POINTS OF INTEREST
Daily rhythmicity is a widespread attribute of living entities, spanning biological kingdoms and functions, as illustrated by just a few examples here.
In unicellular eukaryotes
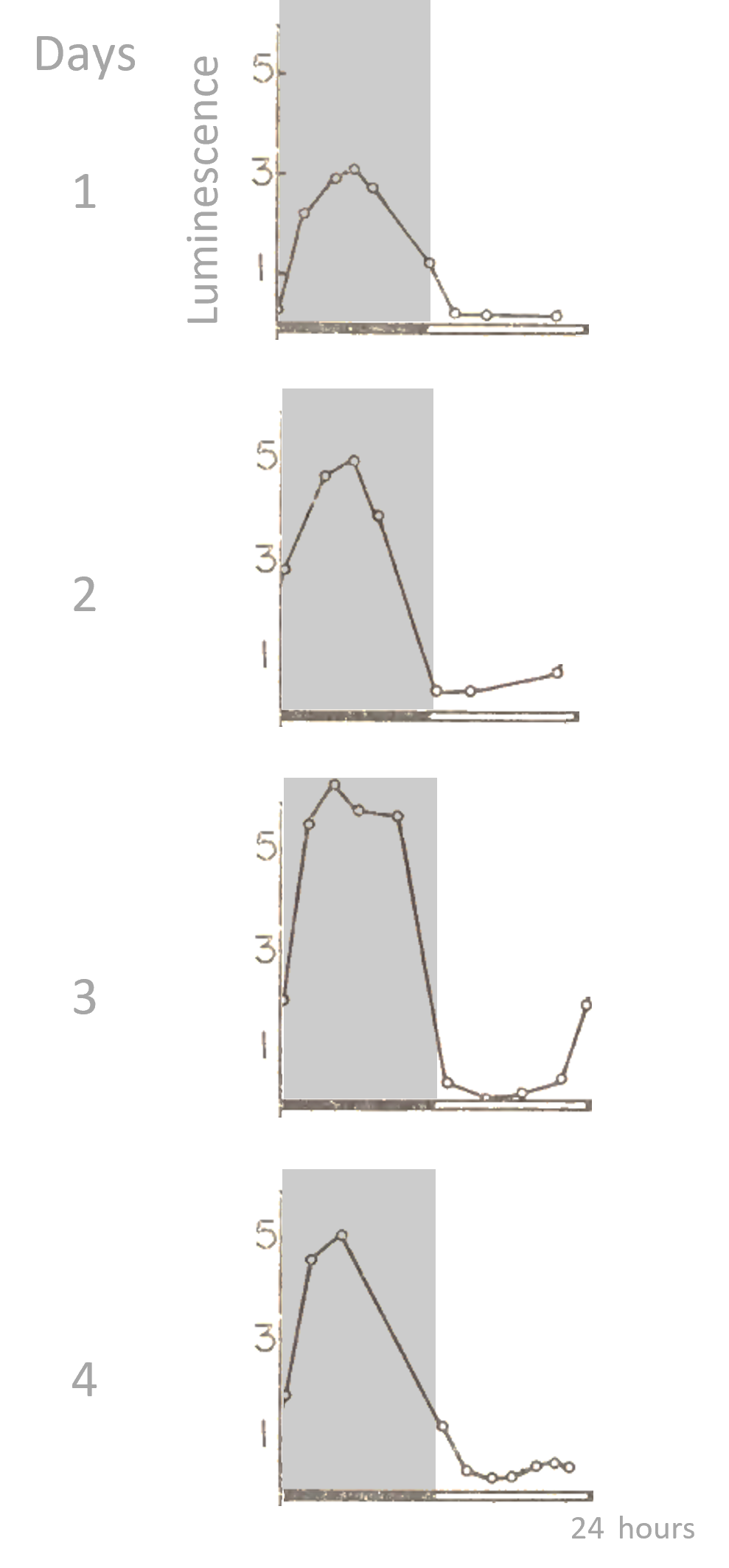
The marine dinoflagellate Gonyaulax polyedra (now Lingulodinium polyedra) expresses a 24-h rhythm of luminescence in culture, with a high level during the dark phase of a 12 h: 12 h light-dark cycle. Black bars and gray shading represent the dark phase, white bars represent the light phase.
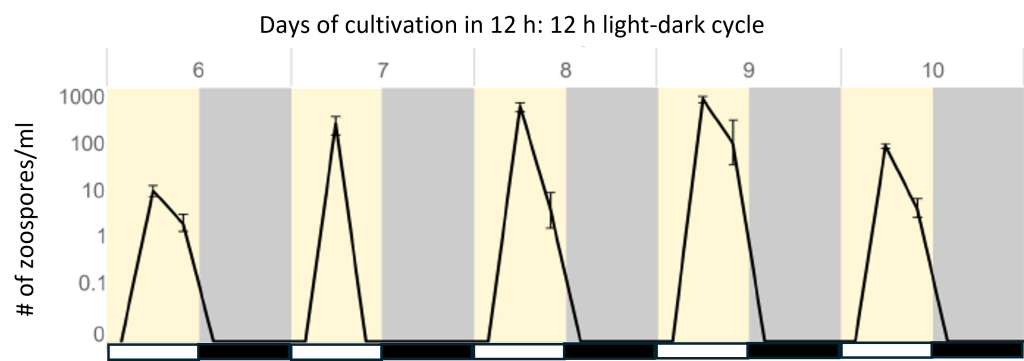
The complex alga Chromera velia exhibits a 24-h rhythm in the formation of its motile stage, zoospores, with a high level during the light phase of a 12 h: 12 h light-dark cycle.
In fungi
The filamentous fungus Neurospora crassa reproduces asexually from spores (conidiation) with a 24-h rhythm when grown in long tubes (“race tubes”) in a 12 h: 12 h light-dark cycle.

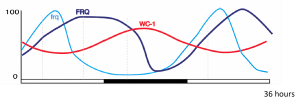
The levels of circadian clock RNAs and proteins involved in the Neurospora core transcription-translation feedback loop oscillate with 24-h rhythms in a 12 h: 12 h light-dark cycle. Shown are frq, frequency RNA; FRQ, FREQUENCY protein; WC-1, WHITE COLLAR-1 protein.
In plants
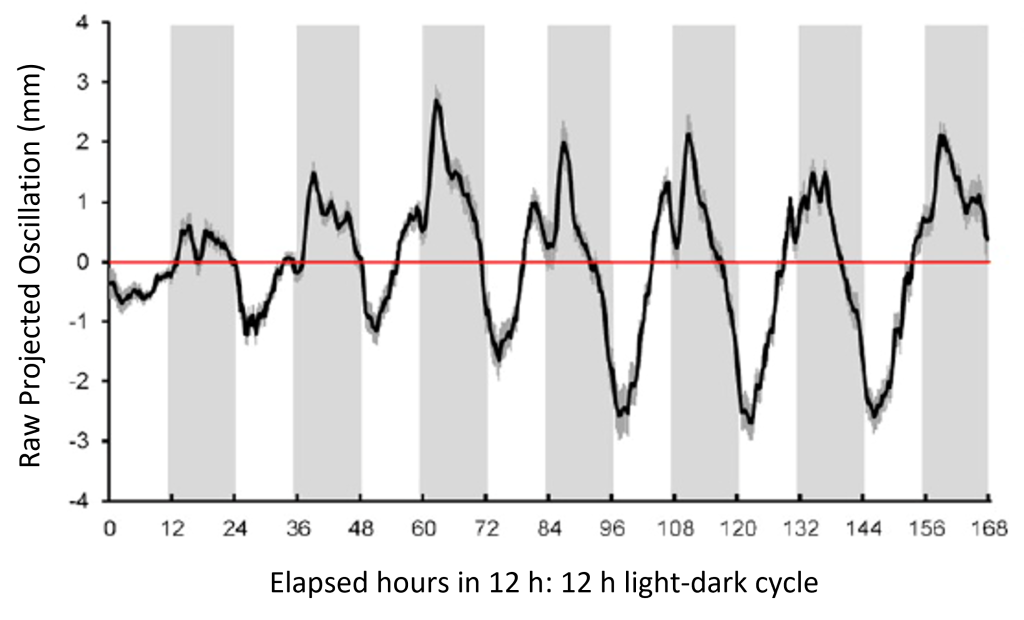
Infrared time-lapse imaging of mature Arabadopsis thaliana plants quantifies a 24-h rhythm of leaf movement in a 12 h: 12 h light-dark cycle (in the detrended plot below, the maximum upright leaf position during the light phase is inverted).
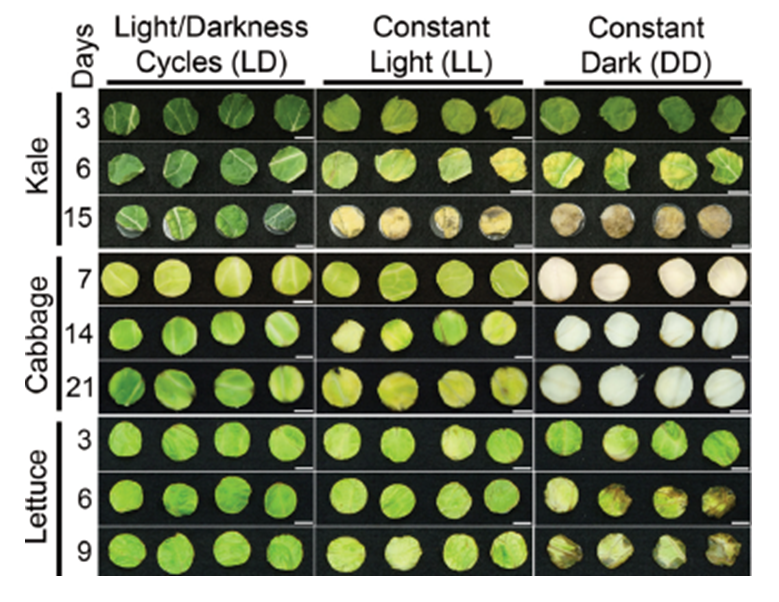
Leaf discs from green vegetables stored after harvest in a 12 h: 12 h light-dark cycle are better preserved than those maintained in constant light or darkness.
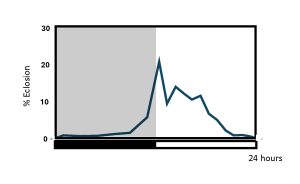
In fruit flies
Pupal eclosion in Drosophila melanogaster occurs mostly during the first half of the light phase in a 12 h: 12 h light-dark cycle, with some anticipation of lights-on.
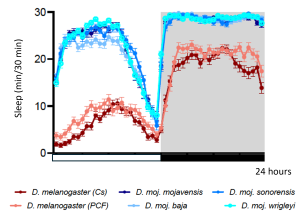
Sleep in stock Drosophila melanogaster and desert-adapted Drosophila mojanvensis strains exhibit 24-h rhythms under laboratory conditions in a 12 h: 12 h light-dark cycle. Cs, Canton-S; PCF, Population Cage Flies.
In rats
Laboratory rats (Rattus norvegicus) singly housed inside a “revolving drum” (an early version of a running wheel) are behaviorally active with a 24-h rhythm, with high levels of activity during the dark phase of a 12 h: 12 h light-dark cycle.

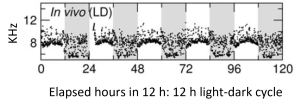
Multi-unit (electrophysiological) activity of the suprachiasmatic nucleus in albino rats (Wistar) oscillates with a 24-h rhythm in vivo, with higher levels during the light phase of a 12 h: 12 h light-dark cycle.
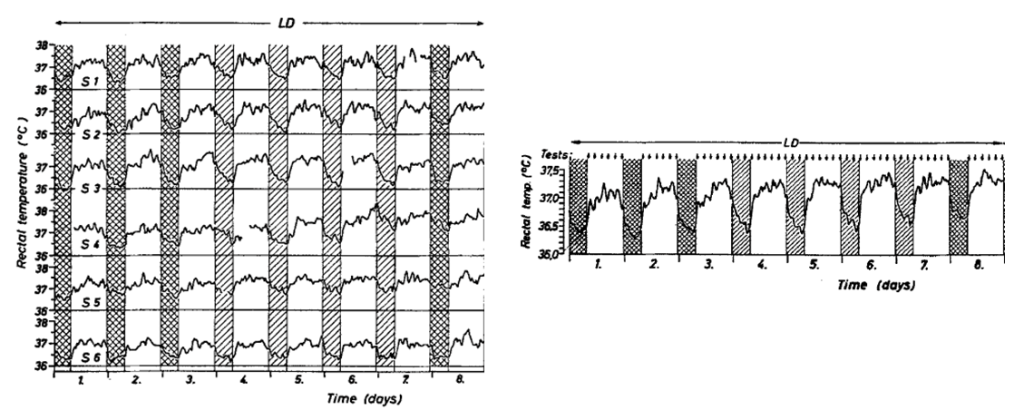
In humans
Rectal temperatures in six male subjects housed in groups of two express a 24-h rhythm when recorded in an isolation facility under a 16 h: 8 h light-dark cycle (individual records, left; mean of six, right).
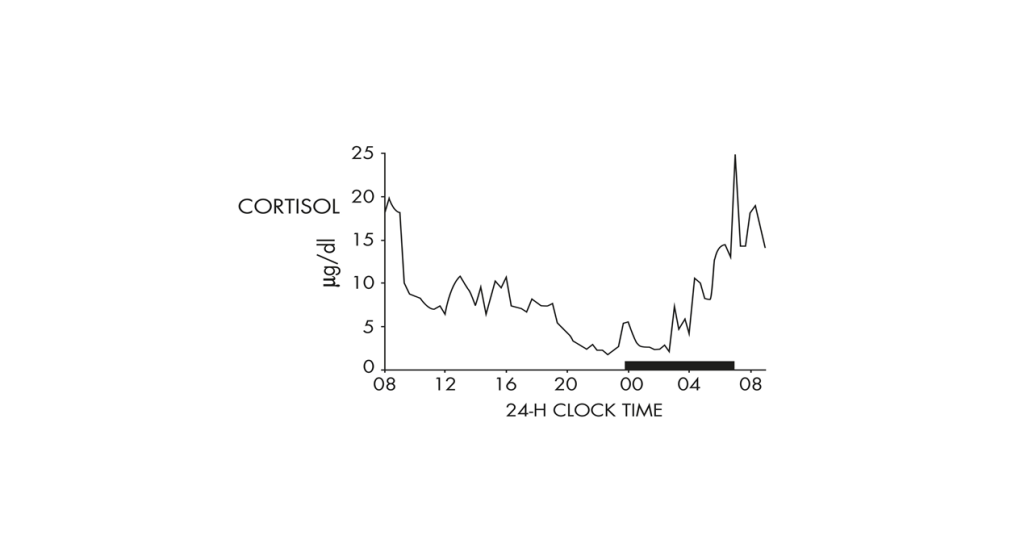
Plasma cortisol levels in a healthy young man under non-restrictive conditions show a 24-h rhythm with an early morning maximum (black bar in this plot is scheduled sleep time).
We acknowledge the large interdisciplinary literature on the mathematics and implementation of diverse algorithms for analyzing data collected over time. While we cannot comprehensively tackle the subject here, we can offer a few points to help you get started. To begin, we recommend a set of two papers as a balanced, practical, and very readable introduction to the use of statistics in biological rhythm research:
Bianchi MT, Phillips AJK, Wang W, Klerman EB. Statistics for sleep and biological rhythms research: From distributions and displays to correlation and causation. J Biol Rhythms 32:7-17, 2017 [doi.org/10.1177/0748730416670050].
Klerman EB, Wang W, Phillips AJK, Bianchi MT. Statistics for sleep and biological rhythms research: Longitudinal analysis of biological rhythms data. J Biol Rhythms 32:18-25, 2017 [doi.org/10.1177/0748730416670051].
Also available from the Society for Research on Biological Rhythms is a helpful list of publicly available tools for time series analysis, at https://srbr.org/education-outreach/research-tools/
The goal of these kinds of tools is to (1) determine the period that best explains the time-dependent variation in a data series, (2) confirm that the rhythmicity detected in the data is statistically greater than attributable to random noise, and (3) characterize other rhythm parameters like phase and waveform. Notably, the approaches traditionally employed in biological rhythm research require that the data are stationary over time (that is, of constant period, amplitude, and waveform from the 1st to the nth cycle), and a few examples of such methods will be mentioned here (for a consideration of non-stationary datasets, see ![]() 2.9 in Chapter 2).
2.9 in Chapter 2).
For data collected over only one or two cycles
Cosinor is a least squares method for analyzing relatively sinusoidal waves by fitting one or more cosine curves to the data, seeking to minimize the residuals between the actual measurement and the fitted model. Assigning a best-fit period allows calculation of rhythm parameters such as amplitude and phase. More complex analyses add a Fourier extension to the single cosine function by incorporating sine and cosine components as harmonics.
For large genome-scale data sets, such as microarrays or RNA-seq, there are special challenges in experimental design and statistical analysis, the solutions to which are still evolving. A consensus statement authored by a who’s-who of 93 prominent circadian biologists lays out the critical issues (Hughes ME, et al., Guidelines for genome-scale analysis of biological rhythms. J Biol Rhythms 32:380-393, 2017 [doi.org/10.1177/074873041772866]).
For data collected over multiple cycles
The chi-square (Sokolove-Bushell) periodogram is a popular method for computing period and its statistical significance, as long as the dataset is composed of equally spaced time points over several cycles (say, 10 at the least). It is an adaptation of the Enright periodogram, based on segmenting the rhythm into equal lengths corresponding to a range of periods, and then overlaying (“folding”) the sections to determine the period at which the amplitude of the resulting waveform is the highest (i.e., when the overlaid peaks and troughs coincide). The Lomb-Scargle periodogram, a Fourier-based method originally applied to astronomical observations, has an added advantage of handling datasets with unequally spaced or even missing time points.
For a discussion of additional methods and their comparative efficacies for the estimation of periodicity, you might consider beginning your reading with:
Sokolove PG, Bushell WN. The chi square periodogram: its utility for analysis of circadian rhythms. J Theor Biol 72:131-160, 1978 [doi.org/10.1016/0022-5193(78)90022-x].
Ruf T. The Lomb-Scargle periodogram in biological rhythm research: Analysis of incomplete and unequally spaced rime-series. Biol Rhythm Res 30:178-201, 2010 [doi.org/10.1076/brhm.30.2.178.1422].
Refinetti R, Cornélissen G, Halberg F. Procedures for numerical analysis of circadian rhythms. Biol Rhythms Res 38:275-325, 2007 [doi.org/10.1080/09291010600903692].
Zielinski T, Moore AM, Troup E, Halliday KJ, Millar AJ. Strengths and limitations of period estimation methods for circadian data. PLoS ONE 9:e96462, 2014 [doi.org/10.1371/journal.pone.0096462].
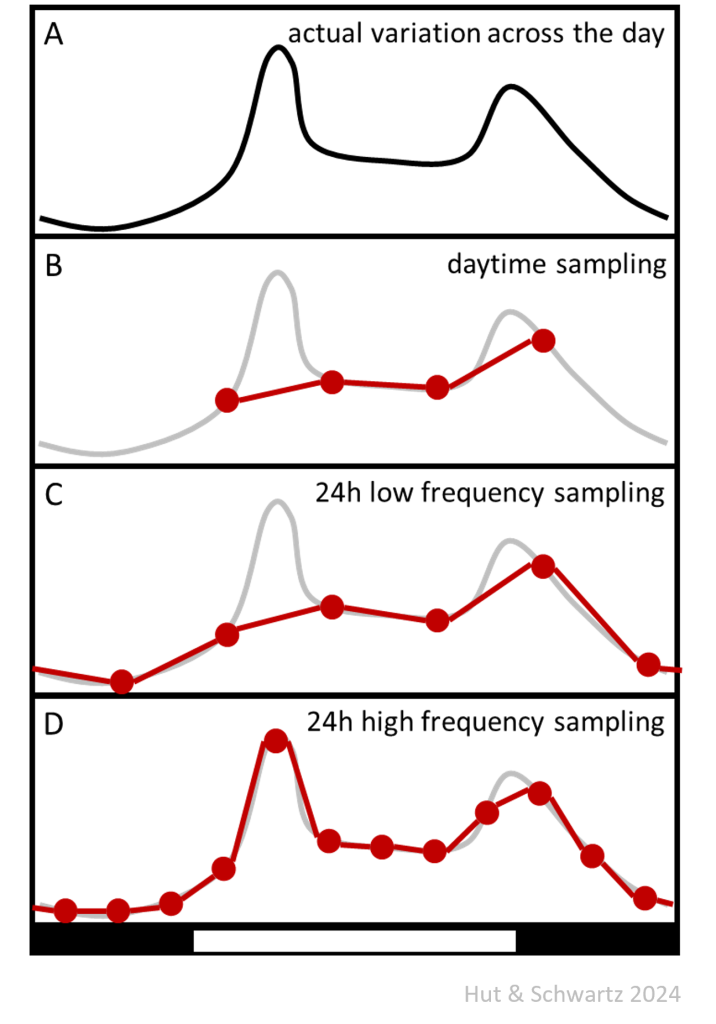
Increasing sampling frequency increases the probability of detecting a rhythm and characterizing its waveform. When only two points per 24-h cycle are sampled, a rhythmic process might not be detected; even when equally spaced, the sampling points may catch the rhythm at its rising and falling phases and thus describe the average value of an otherwise dramatic wave shape. The cartoon below shows how actual rhythm waveform (A) might be mis-interpreted if sampling only takes place during half of the cycle (for example, in the lab during daytime [B]). Sampling across the entire cycle with six equidistant sample points (once every 4 hours) improves the description of the underlying rhythmic shape, but peaks with a width less than the distance between sampling points might be completely missed (C). This leads to the wrong conclusion that the peak of the rhythm is in the early dark phase, whereas increasing the sampling rate to once every two hours (12 sampling points) allows for the interpretation that the peak is actually in the early light phase (D).
In addition to the technical limitations of pen and paper recording instruments 50 years ago (normal paper speed of the Esterline Angus multichannel event recorder was ¾ inch/h), investigators at the time were generally less interested in rhythm amplitude than in period and phase. In large part, this was because the search was on to identify the biological timing mechanism for rhythm generation and its possible localization; even if an experimental manipulation could be found that altered the amplitude of an overt rhythm, it would not be possible to distinguish whether such an effect was acting on the output “hands” downstream of the timer or on its actual “gears.”
And here are two of the acknowledged founders of modern circadian biology engaged in friendly debate, aided by an actogram with period and phase but no amplitude.
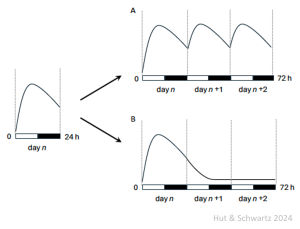
There is an important exception to our statement that the timed sampling of multiple groups beyond 24 h – extending the experiment to 48 h or 72 h or beyond – is not needed to infer daily rhythmicity. The obvious situation is if the population of subjects does not remain in the same biological state over successive 24 h cycles; and a clear case in point would be during development, when a developmental window might extend over several daily cycles.
The following cartoon illustrates the problem. At left, suppose there is variation over the day-night cycle in a measure across multiple groups undergoing development, as denoted by the drawn curve through their mean values. Only by extending the collection could one distinguish between the presence of a daily rhythm on one hand (as in A) or some kind of singular developmental checkpoint on the other (as in B).
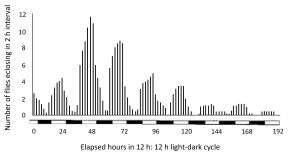
One famous daily rhythm during development is that of Drosophila (example below), when the emergence of adult flies from their pupal cases (eclosion) is timed by an interaction between daily rhythmicity and developmental state. Eclosion is a once-in-a-lifetime event for each individual, but there is a rhythm of the population that extends over several 24-h cycles, gating emergence to the early part of the light phase with some anticipation of lights-on.
For many years investigators searched for a role for rhythmic 24-h geophysical or cosmic timing cues that might serve as alternatives to the existence of an internal circadian clock. Even as late as the second half of the 20th century, such experiments continued – near the South Pole and in geocentric orbit 157 miles above the surface of the Earth – but failed to show such an exogenous factor.
Hamner KC, Finn JC Jr, Sirohi GS, Hoshizaki T, Carpenter BH. The biological clock at the South Pole. Nature 195:476-480, 1962 [doi.org/10.1038/195476a0].
Sulzman FM, Ellman D, Fuller CA, Moore-Ede MC, Wassmer G. Neurospora circadian rhythms in space: a reexamination of the endogenous-exogenous question. Science 225:232-234, 1984 [doi.org/10.1126/science/11540800].
But what about a more prosaic problem: when circadian periods measured in a laboratory with constant light, temperature, humidity, and ad libitum food and water are so close to 24 h that an unrecognized daily cue remains a concern (e.g., an animal technician running the vacuum cleaner in a neighboring room at the same time every morning). Recording the phase of free-running rhythmicity after housing in a reversed light-dark cycle may be one useful test. Another is based on the continuous distribution of free-running periods among individuals; eventually in constant conditions individual rhythms will diverge over time and express a range of differing phases (as long as recording is done individually and independently, avoiding possible social interactions).

The sources of variation impacting the value of 𝝉 are legion: within an individual, at different life stages and circumstances; between individuals within a species, of different sexes and at different latitudes; and across species. For the most part, 𝝉 is revealed under special laboratory conditions; even the extreme cases of constant darkness and constant light in nature do not imply the absence of some kind of Zeitgeber. From an evolutionary point of view, the control of phase, not period, must be the pre-eminent circadian parameter that is under natural selection, because circadian period is not revealed under entrained conditions.
Just a comment here on two other “circa” terms that you will likely see. The term ultradian refers to rhythms with short periods, of several minutes to a few hours; it does not refer to a rhythm with a period less than 24 h but in the circadian range (“circadian range” depends on the organism, light intensity, and other factors, but typically is just a few hours away from 24; see limits of entrainment in Question 2 of this chapter). Ultradian also does not refer to bimodal or more complex 24-h rhythm waveforms driven by the circadian clock, e.g., crepuscular rhythms with activity around dawn and dusk. The term infradian refers to rhythms with long periods, typically monthly or annually; it does not refer to a rhythm with a period greater than 24 h but in the circadian range.
The origins of circadian biology lie in studies of the daily (nyctinastic) movements of leaves and petals. Although such movements had been known for millennia, Jean-Jacques d’Ortous de Mairan (1678 – 1771) is given credit for reporting (in 1729) that the daytime opening and nighttime closing of Mimosa leaves continued when the plant was placed in a dark place, “sensing” the sun without seeing it. His observation was published as a short News and Views-like communication (in 1731) that was actually not written by him. It was the Swiss botanist Augustin Pyramus de Candolle (1778 – 1841) who later focused his investigations on light: by reversing the light-dark cycle he inverted the leaf movement rhythm, and, by placing the plants in constant light, he reported that a persistent rhythm was shorter than 24 h and varied from plant to plant. He concluded that the movements “…are linked to a disposition to periodic movement inherent to plants…” (in 1832). Nearly 100 years of debate and experimentation had to pass, including perfection of technique and instrumentation, before a skeptical Wilhelm Friedrich Philipp Pfeffer (1845 – 1920), the pre-eminent plant physiologist of his time, clearly demonstrated persistent, endogenous, free-running rhythms of leaf movements in bean plants housed in constant light (in 1915).
For a scholarly and enjoyable review of the early botanical origins of circadian biology, see:
Emery P, Klarsfeld A, Stanewsky R, Shafer OT. Sensitive timing: A reappraisal of chronobiology’s foundational texts. J Biol Rhythms 38:245-258, 2023 [doi.org/10.1177/07487304231169080].
Changing the intensity of constant light may increase or decrease 𝝉, depending on the organism (Daan and Pittendrigh, 1976; and see ![]() 2.8, below), but it generally also destabilizes rhythmicity in mammals, especially at higher intensities. The result may be frank arrhythmicity or a phenomenon known as “splitting,” when an animal’s normally consolidated activity/rest interval separates into two activity bouts that free-run with different periods for a number of cycles and then stably re-synchronize with a common period but 180° in antiphase (12 circadian hours apart). These phenomena appear to be due to aberrant intercellular coupling within the SCN, either by desynchronizing cellular rhythms from each other (resulting in behavioral arrhythmicity) or by reorganizing the left and right sides of the SCN to oscillate in antiphase (resulting in splitting) (de la Iglesia et al., 2000; Ohta et al., 2005; Yan et al., 2005). Given the SCN’s central role in driving, gating, or synchronizing a host of brain and body rhythms, constant light’s disruptive effects on timing can be multi-dimensional (including the real-world effects of artificial light at night).
2.8, below), but it generally also destabilizes rhythmicity in mammals, especially at higher intensities. The result may be frank arrhythmicity or a phenomenon known as “splitting,” when an animal’s normally consolidated activity/rest interval separates into two activity bouts that free-run with different periods for a number of cycles and then stably re-synchronize with a common period but 180° in antiphase (12 circadian hours apart). These phenomena appear to be due to aberrant intercellular coupling within the SCN, either by desynchronizing cellular rhythms from each other (resulting in behavioral arrhythmicity) or by reorganizing the left and right sides of the SCN to oscillate in antiphase (resulting in splitting) (de la Iglesia et al., 2000; Ohta et al., 2005; Yan et al., 2005). Given the SCN’s central role in driving, gating, or synchronizing a host of brain and body rhythms, constant light’s disruptive effects on timing can be multi-dimensional (including the real-world effects of artificial light at night).
Daan S, Pittendrigh CS. A functional analysis of circadian pacemakers in nocturnal rodents. III. Heavy water and constant light: homeostasis of frequency? J Comp Physiol A 106:267-290, 1976 [doi.org/10.1007/BF01417858].
de la Iglesia HO, Meyer J, Carpino A Jr, Schwartz WJ. Antiphase oscillation of the left and right suprachiasmatic nuclei. Science 290:799-801, 2000 [doi.org/10.1126/science.290.5492.799].
Ohta H, Yamazaki S, McMahon DG. Constant light desynchronizes mammalian clock neurons. Nat Neurosci 8:267-269, 2005 [doi.org/10.1038/nn1395].
Yan L, Foley NC, Bobula JM, Kriegsfeld LJ, Silver R. Two antiphase oscillations occur in each suprachiasmatic nucleus of behaviorally split hamsters. J Neurosci 25:9017-9026, 2005 [doi.org/10.1523/JNEUROSCI.2538-05.2005].
Named after Arnold Eskin (1940 – 2019), who acknowledged in his 1979 paper that his diagram was inspired by an earlier 1957 schematic by Pittendrigh and Victor Bruce that was published in the proceedings of a symposium.
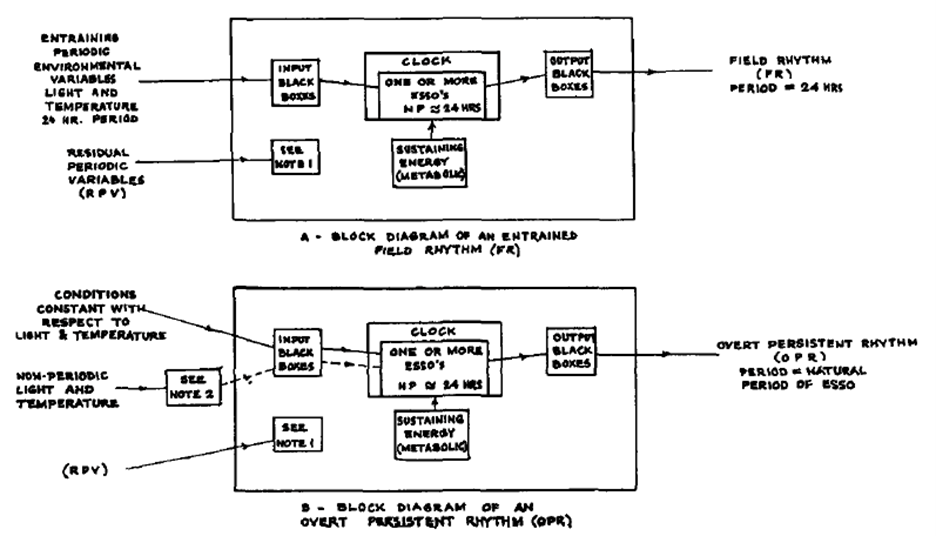
Notes 1 and 2 were defined in the text of the article:
Note 1: All periodically fluctuating environmental variables other than light and temperature are
called residual periodic variables (RPV). They are assumed to be never coupled to the oscillator.
Note 2: Light shocks, temperature shocks, and non-periodic treatment with light and temperature may affect the phase of OPR.
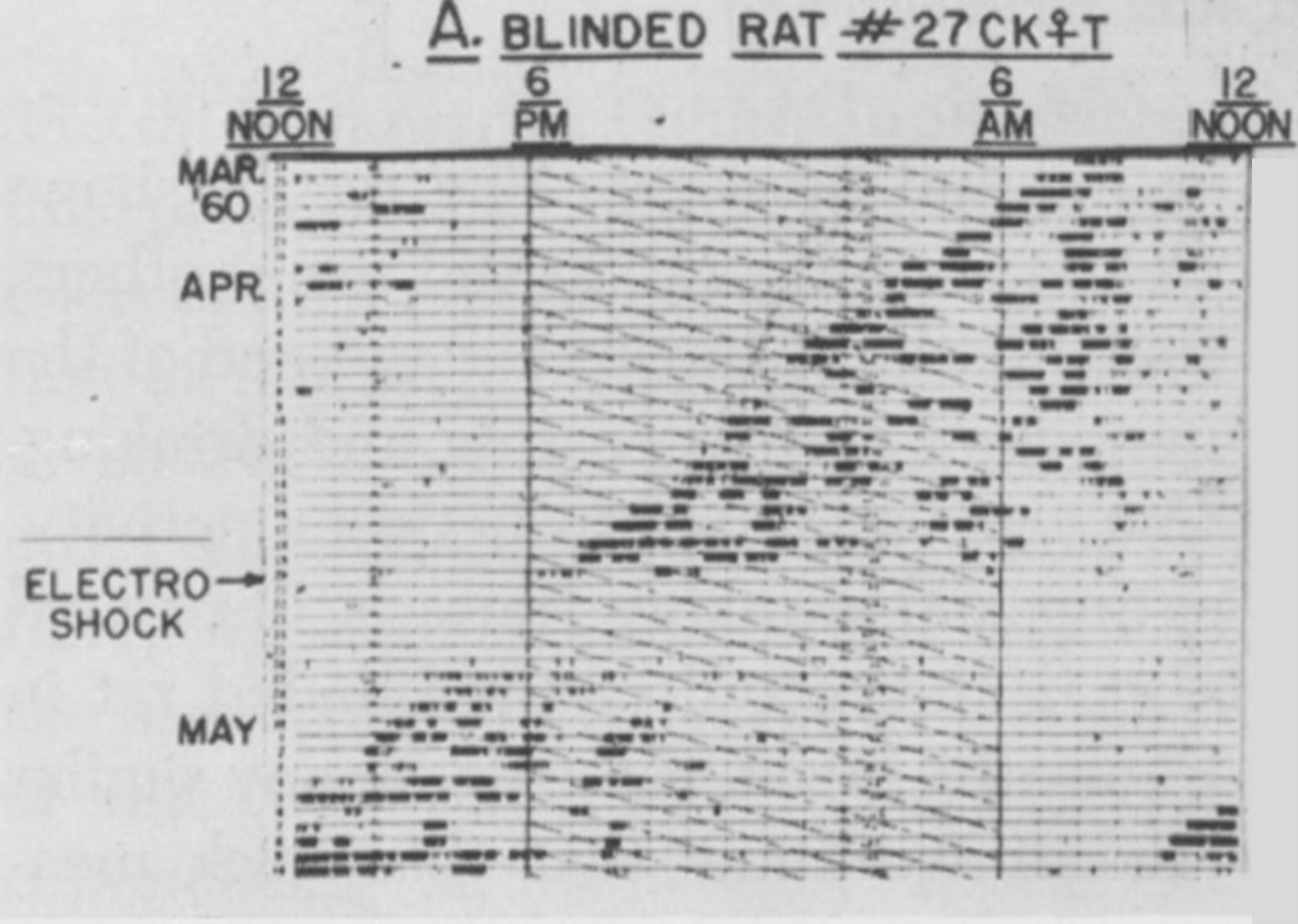
One example of the non-identity of the pacemaker and a rhythm that it drives is shown in an experiment by Curt Richter (1894 – 1998) below, with the reversible inactivation of locomotor (running wheel) activity for several days after an experimental perturbation (electric shock). Upon recovery, it is clear that the pacemaker has continued to run unperturbed but unseen through the shock, which apparently affected only the pacemaker’s “hands” (output) but spared its “gears” (internal timekeeping mechanism). Richter obtained a similar result using 6 h of nitrous oxide inhalation as the experimental perturbation.
In reality, the Eskinogram arrow from the pacemaker to the output rhythm is not unidirectional; as an example, how we assay the locomotor activity rhythm can impact the expression of the rhythm itself, including the daily rhythm’s shape and the free-running rhythm’s period.
To our knowledge, the first report of the Drosophila locomotor activity rhythm was in 1956 (de F Roberts, 1956); in that report, 6 male and 6 female D. robusta were housed in a common chamber with a grid of fine wires, revealing a daily population rhythm with activity restricted to a few hours before lights-off (upper panel below, of four consecutive 24-h days in a 12 h: 12 h light-dark cycle). Now Drosophila locomotor activity is usually monitored using the Drosophila Activity Monitoring (DAM) System (TriKinetics, Waltham, Massachusetts), wherein individual flies are housed in glass tubes and activity is recorded over days via infrared beam breaks, yielding instead bimodal peaks around lights-on and lights-off that anticipate the lighting transitions (lower panel below, quadruple plotted for comparison).
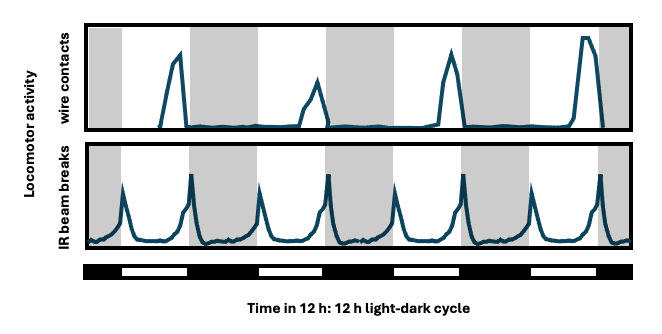
Lower panel, re-drawn from Fig. 1 in: Allada R, Chung B. Circadian organization of behavior and physiology in Drosophila. Ann Rev Physiol 72:605-624, 2010 [doi.org/10.1146/annurev-physiol-021909-135815].
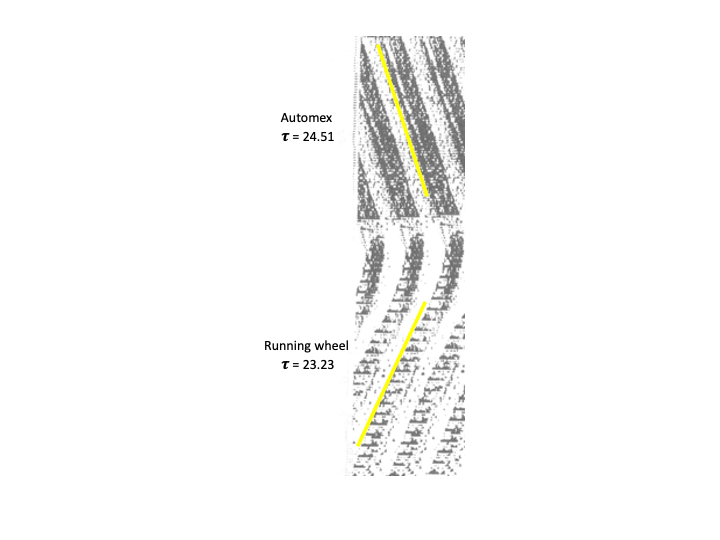
And in 6-week old rats, blinded at birth by bilateral ocular enucleation, the value of 𝝉 is shorter for rats with a running wheel than that for rats without a wheel (the latter with their locomotor activity recorded via an electromagnetic animal activity monitor [Automex system, Columbus Instruments, Columbus, Ohio.]). In the figure below, the yellow stripes are added for visualizing 𝝉 via eye fit lines through activity onsets. The greater the wheel revolutions/day, the shorter the 𝝉 (Shiori et al., 1991).
Shioiri T, Takahashi K, Yamada N, Takahashi S. Motor activity correlates negatively with free-running period, while positively with serotonin contents in SCN in free-running rats. Physiol Behav 49:779-786, 1991 [doi.org/10.1016/0031-9384(91)90318-I].
Coined by Pittendrigh, “Aschoff’s Rule” is based on Aschoff’s empirical observations of the relationship between the intensity of constant light and the value of 𝝉 in diurnally- and nocturnally-active animals. For diurnal animals, increasing light intensity tends to shorten 𝝉 of their free-running behavioral rhythm, while it is the opposite for nocturnal animals, with increasing intensity lengthening 𝝉. Although there are exceptions, the rule is part of the evidence for a theory of photic entrainment based on tonic (parametric) effects of light (see discussion in Question 2 of this chapter).
A helpful introduction to the analysis of data sets with variable periods and amplitudes, rhythms at multiple time scales, noise, and trend over time is:
Leise TL. Analysis of nonstationary time series for biological rhythms research. J Biol Rhythms 32:187-194, 2017 [doi.org/10.1177/0748730417709105].
Of particular interest is the utility of wavelet transforms, which do not rely on fixed sines and cosines for the decomposition of time series. Discrete transforms can extract a circadian component in the presence of discontinuities, noise, and trend; and continuous transforms can quantify variability in period and amplitude over time. Some starting references for circadian applications of wavelets include:
Leise TL, Harrington ME. Wavelet-based time series analysis of circadian rhythms. J Biol Rhythms 26:454-463, 2011 [doi.org/10.1177/0748730411416330].
Leise TL, Indic P, Paul MJ, Schwartz WJ. Wavelet meets actogram. J Biol Rhythms 28:62-68, 2013 [doi.org/10.1177/0748730412468693].
Leise TL. Wavelet-based analysis of circadian behavioral rhythms. Methods in Enzymology 551:95-119, 2015 [doi.org/10.1016/bs.mie.2014.10.011].
Here are a few reviews as starting points to enter the literature on molecular and genetic analyses of circadian clocks in model organisms:
Tataroglu O, Emery P. The molecular ticks of the Drosophila circadian clock. Curr Opin Insect Sci 7:51-57, 2015 [doi.org/10.1016/j.cois.2015.01.002].
Takahashi JS. Transcriptional architecture of the mammalian circadian clock. Nat Rev Genetics
18:164-179, 2017 [doi.org/10.1038/nrg.2016.150].
Swann JA, Golden SS, LiWang A, Partch CL. Structure, function, and mechanism of the core circadian clock in cyanobacteria. J Biol Chem 293:5026-5034, 2018 [doi.org/10.1074/jbc.TM117.001433].
Loros JJ. Principles of the animal molecular clock learned from Neurospora. Eur J Neurosci 51:19-33, 2020 [doi.org/10.1111/ejn.14354].
Nakamichi N. The transcriptional network in the Arabidopsis circadian clock system. Genes 11:1284, 2020 [doi.org/10.3390/genes11111284].
Here are a few reviews as starting points to enter the literature on the suprachiasmatic nucleus:
Weaver DR. The suprachiasmatic nucleus: a 25-year retrospective. J Biol Rhythms 13:100-112, 1998 [doi.org/10.1177/074873098128999952].
Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: cell autonomy and network properties. Annu Rev Physiol 72:551-577, 2010 [doi.org/10.1146/annurev-physiol-021909-135919].
Ono D, Weaver DR, Hastings MH, Honma K-I, Honma S, Silver R. The suprachiasmatic nucleus at 50: Looking back, then looking forward. J Biol Rhythms 39:135-165, 2024 [doi.org/10.1177/07487304231225706].
The circadian phenotypes of mutated mice are strongly influenced by genetic background, as a few examples can illustrate. While mouse per2 knockouts congenic with the 129sv strain lead to arrhythmicity (Zheng et al., 1999; Bae et al., 2001), knockouts congenic with C57BL/6J do not (Xu et al., 2007; Pendergast et al., 2010). And the pathologically lengthened free-running period of the clock∆19 mutation, initially induced on a C57BL/6J background, is suppressed by crosses with BALB/cJ or C3H/HeJ strains (Shimomura et al., 2010, 2013). The translational implications of such background effects are consequential: while clock∆19/∆19 on C57BL/6J develops high fat diet-induced obesity, the mutation on ICR mice does not (in fact, the mice lose weight; Oishi et al., 2006).
Zheng B, Larkin DW, Albrecht U, Sun ZS, Sage M, Eichele G, Lee CC, Bradley A. The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature 400:169-173, 1999 [doi.org/10.1038/22118].
Bae K, Jin X, Maywood ES, Hastings MH, Reppert SM, Weaver DR. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron 30:525-536, 2001 [doi.org/10.1016/s0896-6273(01)00302-6].
Xu Y, Toh KL, Jones CR, Shin J-Y, Fu Y-H, Ptáček LJ. Modeling of a human circadian mutation yields insights into clock regulation by PER2. Cell 128:59-70, 2007 [doi.org/10.1016/j.cell.2006.11.043].
Pendergast JS, Friday RC, Yamazaki S. Distinct functions of Period2 and Period3 in the mouse circadian system revealed by in vitro analysis. PLoS ONE 5:e8552, 2010 [doi.org/10.1371/journal.pone.0008552].
Shimomura K, Lowrey PL, Vitaterna MH, Buhr ED, Kumar V, Hanna P, Omura C, Izumo M, Low SS, Barrett RK, LaRue SI, Green CB, Takahashi JS. Genetic suppression of the circadian Clock mutation by the melatonin biosynthesis pathway. Proc Natl Acad Sci USA 107:8399-8403, 2010 [doi.org/10.1073/pnas.1004368107].
Shimomura K, Kumar V, Koike N, Kim T-K, Chong J, Buhr ED, Whiteley AR, Low SS, Omura C, Fenner D, Owens JR, Richards M, Yoo S-H, Hong H-K, Vitaterna MH, Bass J, Pletcher MT, Wiltshire T, Hogenesch J, Lowrey PL, Takahashi JS. Usf1, a suppressor of the circadian Clock mutant, reveals the nature of the DNA-binding of the CLOCK:BMAL1 complex in mice. eLife 2:e00426, 2013 [doi.org/10.7554/eLife.00426].
Oishi K, Atsumi G-I, Sugiyama S, Kodomari I, Kasamatsu M, Machida K, Ishida N. Disrupted fat absorption attenuates obesity induced by a high-fat diet in Clock mutant mice. FEBS Lett 580:127-130, 2006 [doi.org/10.1016/j.febslet.2005.11.063].
Manifestly normal circadian function may belie underlying genetic disruption. Examples of such compensation can occur at multiple biological levels, including the up-regulation of gene paralogs (Baggs et al., 2009) and the presence of intercellular coupling within neural circuits (Liu et al., 2007).
Baggs JE, Price TS, DiTacchio L, Panda S, FitzGerald GA, Hogenesch JB. Network features of the mammalian circadian clock. PLoS Biology 7:e1000052, 2009 [doi.org/10.1371/journal.pbio.1000052].
Liu AC, Welsh DK, Ko CH, Tran HG, Zhang EE, Priest AA, Buhr ED, Singer O, Meeker K, Verma IM, Doyle FJ III, Takahashi JS, Kay SA. Intercellular coupling confers robustness against mutations in the SCN circadian clock network. Cell 129:605-616, 2007 [doi.org/10.1016/j.cell.2007.02.047].
Pathological mechanisms and even pathological states ascribed to the experimental inactivation of the circadian molecular feedback loop may depend on how the loop is broken, whether by disrupting genes in the activating (clock, bmal1) vs. the repressing (per, cry) limbs of the loop. For example, either bmal1 or per loss is associated with pathological defects in murine wound healing, but the underlying mechanisms are different: the bmal1 knockout results in insufficient proliferation of fibroblasts or keratinocytes, while per loss leads to hyperproliferation with an excess of fibroblasts and polymorphonuclear cells (Kowalska et al., 2013). Even more dramatic are the results of bmal1 or per loss on brain pathology, with signs of neural degeneration in the former but not in the latter (Musiek et al., 2013). As a counter-example, abnormal glucose homeostasis follows either bmal1 or per loss, suggesting that this pathology is indeed a consequence of disrupted circadian rhythmicity per se (Lamia et al., 2008).
Kowalska E, Ripperger JA, Hoegger DC, Bruegger P, Buch T, Birchler T, Mueller A, Albrecht U, Contaldo C, Brown SA. NONO couples the circadian clock to the cell cycle. Proc Natl Acad Sci USA 110: 1592-1599, 2013 [doi.org/10.1073/pnas.1213317110].
Musiek ES, Lim MM, Yang G, Bauer AQ, Qi L, Lee Y, Roh JH, Ortiz-Gonzalez X, Dearborn JT, Culver JP, Herzog ED, Hogenesch JB, Wozniak DF, Dikranian K, Glasson BI, Weaver DR, Holtzman DM, FitzGerald GA. Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. J Clin Invest 123:5389-5400, 2013 [doi.org/10.1172/JCI70317].
Lamia KA, Storch K-F, Weitz CJ. Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci USA 105:15172-15177, 2008 [doi.org/10.1073/pnas.0806717105].
Scheer FAJL, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA 106:4453-4458, 2009 [doi.org/10.1073/pnas.0808180106].
Wang W, Yuan RK, Mitchell JF, Zitting K-M, St. Hilaire MA, Wyatt J, Scheer FAJL, Wright KP Jr, Brown EN, Ronda JM, Klerman EB, Duffy JF, Dijk D-J, Czeisler CA. Desynchronizing the sleep-wake cycle from circadian timing to assess their separate contributions to physiology and behaviour and to estimate intrinsic circadian period. Nature Protocols 18:579-603, 2023 [doi.org/10.1038/s41596-022-00746-y].
Masking is characterized as either positive (enhancing) or negative (inhibiting), although such categorization depends on the measure chosen. For example, light is known to inhibit the expression of locomotor activity in nocturnal rodents (negative) while simultaneously enhancing features of sleep (positive). Different neural pathways may underlie the locomotor-suppressing vs. sleep-inducing effects of the masking light pulse.
For a helpful review on the nature of masking, see:
Mrosovsky N. Masking: history, definitions, and measurement. Chronobiol Int 16:415-429, 1999 [doi.org/10.3109/07420529908998717].
One adaptive benefit of an internal timekeeping mechanism is the capacity to anticipate a recurring, periodic event, like sunrise. As one example, plants like Arabidopsis increase their expression of photosynthesis-related genes well before the daily dark to light transition occurs. When plants are rendered arrhythmic by constitutive overexpression of the clock gene CCA1, the transgenic mutant remains responsive to light, but it fails to anticipate its periodic re-occurrence and appears less fit than wild type when tested under certain laboratory conditions (Green et al., 2002). And young heliotropic sunflower plants track the sun as it moves from east to west and then reorient overnight by a circadian mechanism to face the east in anticipation of the coming dawn (Atamian et al., 2016); warming of the flowers by the rising sun appears to increase pollinator visits in the early morning hours.
Also, a wide array of animals can anticipate periodic mealtimes, and research on rodents indicates that such “food anticipatory activity” represents the action of a circadian oscillator (“food entrainable oscillator” [FEO]) that is not the SCN (Stephan 2002); its identity remains under active investigation (Crosby et al., 2019; Petersen et al., 2022; Ehichioya et al., 2023), as well as its relationship to a “methamphetamine sensitive circadian oscillator” (Taufique et al., 2022).
Green RM, Tingay S, Wang Z-Y, Tobin EM. Circadian rhythms confer a higher level of fitness to Arabidopsis plants. Plant Physiol 129:576-584, 2002 [doi.org/10.1104/pp.004374].
Atamian HS, Creux NM, Brown RI, Garner AG, Blackman BK, Harmer SL. Circadian regulation of sunflower heliotropism, floral orientation, and pollinator visits. Science 353:587-590, 2016 [doi.org/10.1126/science.aaf9793].
Stephan FK. The “other” circadian system: food as a zeitgeber. J Biol Rhythms 17:284-292, 2002 [doi.org/10.1177/074873040201700402].
Crosby P, Hamnett R, Putker M, Hoyle NP, Reed M, Karam CJ, Maywood ES, Stangherlin A, Chesham JE, Hayter EA, Rosenbrier-Ribeiro L, Newham P, Clevers H, Bechtold DA, O’Neill JS. Insulin/IGF-1 drives PERIOD synthesis to entrain circadian rhythms with feeding time. Cell 177:896-909, 2009 [doi.org/10.1016/j.cell.2019.02.017].
Petersen CC, Cao F, Stinchcombe AR, Mistlberger RE. Multiple entrained oscillator model of food anticipatory rhythms. Sci Rep 12:9306, 2022 [doi.org/10.1038/s41598-022-13242-w].
Ehichioya DE, Taufique SKT, Farah S, Yamazaki S. A time memory engram embedded in a light-entrainable circadian clock. Curr Biol 33:5233-5239, 2023 [doi.org10.1016/j.cub.2023.10.027].
Taufique SKT, Ehichioya DE, Pendergast JS, Yamazaki S. Genetics and functional significance of the understudied methamphetamine sensitive circadian oscillator (MASCO). F1000 Research 11:1018, 2022 [doi.org/10.12688/f1000research.125432.2].
Perhaps not unexpectedly, there are significant, qualitative differences in locomotor activity rhythms expressed by animals when they are moved from the laboratory to natural conditions of light and temperature outdoors. Drosophila melanogaster under such “semi-natural” environments (they remain housed in glass tubes) show differences in the timing of their morning and evening activity bouts, and their diurnal siesta in the lab is replaced by a prominent afternoon activity bout outdoors (Vanin et al., 2012; Menegazzi et al., 2012; De et al., 2013).
Vanin S, Bhutani S, Montelli S, Menegazzi P, Green EW, Pegoraro M, Sandrelli F, Costa R, Kyriacou CP. Unexpected features of Drosophila circadian behavioural rhythms under natural conditions. Nature 484:371-375, 2012 [doi.org/10.1038/nature10991].
Menegazzi P, Yoshii T, Helfrich-Förster C. Laboratory versus Nature: the two sides of the Drosophila circadian clock. J Biol Rhythms 27:433-442, 2012 [doi.org/10.1177/0748730412463181].
De J, Varma V, Saha S, Sheeba V, Sharma VK. Significance of activity peaks in fruit flies, Drosophila melanogaster, under seminatural conditions. Proc Natl Acad Sci USA 110:8984-8989, 2013 [doi.org/10.1073/pnas.1220960110].
Notably, our dependable nocturnal laboratory rodents may not be so reliably nocturnal outdoors. Golden spiny mice (Acomys russatus) are nocturnal when housed in the laboratory but become diurnal within a day after transfer to 1000 m2 enclosures in the Judean desert (Levy et al., 2007). Their immediate transition to diurnality in the field suggests a masking mechanism downstream of the circadian pacemaker (presumably the pacemaker in the SCN). Male and female laboratory mice housed together in outdoor experimental pens in Western Russia for two years – where they were exposed to natural weather conditions & aerial predation and competed among themselves for food, water, shelter, and mating – exhibited variable feeding rhythms that were not restricted to the night (Daan et al., 2011). And even the golden hamster (Mesocricetus auratus), the archetypical nocturnal rodent species in the laboratory, is not really nocturnal in its natural habitat in southern Turkey, as it assumes a more crepuscular-like pattern (Gattermann et al., 2008).
Levy O, Dayan T, Kronfeld-Schor N. The relationship between the golden spiny mouse circadian system and its diurnal activity: an experimental field enclosures and laboratory study. Chronobiol Int 24:599-613, 2007 [doi.org/10.1080/07420520701534640].
Daan S, Spoelstra K, Albrecht U, Schmutz I, Daan M, Daan B, Rienks F, Poletaeva I, Dell’Omo G, Vyssotski A, Lipp H-P. Lab mice in the field: unorthodox daily activity and effects of a dysfunctional circadian clock allele. J Biol Rhythms 26:118-129, 2011 [doi.org/10.1177/0748730410397645].
Gattermann R, Johnston RE, Yigit N, Fritzsche P, Laarimer S, Özkurt S, Neumann K, Song Z, Colak E, Johnston J, McPhee ME. Golden hamsters are nocturnal in captivity but diurnal in nature. Biol Lett 4:253-255, 2008 [doi.org/10.1098/rsbl.2008.0066].
Unlike in non-mammalian vertebrates, 24-h entrainment of the circadian locomotor activity rhythm in adult mammals depends on intact ocular photoreception; the rhythm free runs after bilateral orbital enucleation (but see ![]() 2.42). Moreover, when eight rodent species were blinded and housed outdoors at the University of California Berkeley Field Station, exposure to other 24-h cues – temperature, humidity, noise, and so on – failed as effective Zeitgebers. However, the natural environment is admittedly complex, and certain biotic factors may well act as effective non-photic Zeitgebers (Bussi et al., 2024).
2.42). Moreover, when eight rodent species were blinded and housed outdoors at the University of California Berkeley Field Station, exposure to other 24-h cues – temperature, humidity, noise, and so on – failed as effective Zeitgebers. However, the natural environment is admittedly complex, and certain biotic factors may well act as effective non-photic Zeitgebers (Bussi et al., 2024).
Nelson RJ, Zucker I. Absence of extraocular photoreception in diurnal and nocturnal rodents exposed to direct sunlight. Comp Biochem Physiol 69A:145-148, 1981 [doi.org/10.1016/0300-9629(81)90651-4].
Bussi IL, Ben-Hamo M, Salazar Leon LE, Casiraghi LP, Zhang VY, Neitz AF, Lee J, Takahashi JS, Kim JJ, de la Iglesia HO. The circadian molecular clock in the suprachiasmatic nucleus is necessary but not sufficient for fear entrainment. Proc Natl Acad Sci USA 121:e2316841121, 2024 [doi.org/10.1073/pnas.2316841121].
To the best of our knowledge, the first “modern” actogram was conceived by Maynard S. Johnson (1900 – 1981). Johnson graduated from Bates College, Lewiston, Maine, U.S.A. in 1921 with a Bachelor of Arts in Zoology. The 1921 Bates yearbook described him as “A Progeny of Learning” and also detailed in part “Maynard is not avaricious but he does love lady scientists’ fudge. He is ready to try anything or anyone once, from a moonlight waltz to a sermon….” (incidentally, we have failed in our attempts to identify “fudge” as a 1920’s-era slang term for anything other than a chocolate confection).
He was awarded a Ph.D. in Zoology from the University of Illinois in 1925, and a paper was published in 1926 (Johnson, 1926). In it he described the rhythmic locomotor activity of forest deer mice (Peromyscus leucopus). He housed each mouse in a cage suspended by rubber bands, and an indicator attached to the cage traced cage movement on a paper disk rotated by an alarm clock over 12 h. When the mouse was quiet, the record was smooth; when active, the record was irregular (Johnson’s Figure 1 below shows such a tracing).
Then, without any explanation for how he decided on the design of his plots, he wrote “There was fairly constant activity at night, and nearly continuous quiet during the day. The time when activity began and ceased agreed very closely with the time of arrival of darkness and light, as may be seen from figure 2. In this figure, periods of activity are shown by blocks of black, and periods of quiet by single lines. The heavy vertical line shows time of sunset. A study of the figure shows a relation between the seasons and time of activity. In the winter, when the days are short and the nights are long, the daily period of quiet is shorter and of activity is longer.” His Figure 2 (below) represents the first bona fide actograms.
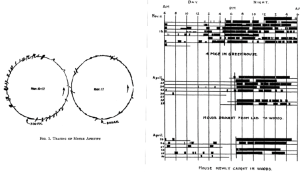
In the same paper, he presented an actogram showing persistence of the rhythm in constant darkness, then rhythm re-entrainment with reversed lighting, followed again by constant darkness.
In 1939 a second laboratory-based paper appeared (Johnson, 1939), this time from the Biological Laboratories of Harvard University. His official position at Harvard is unclear and lasted for only two years (in his paper, he thanked Drs. H.W. Rand and T.J.B. Stier for laboratory space and equipment; Stier had published a paper on the thermoregulatory function of mouse locomotor activity in 1933). Johnson tested the effects of different intensities of constant light on free-running period, demonstrating what eventually would become part of Aschoff’s Rule (recall ![]() 2.8), and Johnson concluded that “…this animal has an exceptionally substantial and durable self-winding and self-regulating physiological clock, the mechanism of which remains to be worked out…” He soon left academia for a post with the newly created U.S. Fish and Wildlife Service, and he later served in World War II and afterwards throughout Asia working on pest control.
2.8), and Johnson concluded that “…this animal has an exceptionally substantial and durable self-winding and self-regulating physiological clock, the mechanism of which remains to be worked out…” He soon left academia for a post with the newly created U.S. Fish and Wildlife Service, and he later served in World War II and afterwards throughout Asia working on pest control.
Johnson MS. Effect of continuous light on periodic spontaneous activity of white-footed mice (Peromyscus). J Exp Zool 82:315-328, 1939 [doi.org/10.1002/jez.1400820209].
It’s interesting that the differential speed of photic re-entrainment – with advancing phase shifts proceeding more slowly than delaying ones – seems to be a general feature of circadian systems. Pittendrigh et al. in 1958 noted (p. 972), “Finally, we note that there is strong evidence in hamsters, cockroaches, and birds that advancing transients persist for many more cycles than do delaying transients; in other words, the whole system is more easily reset by a “dawn” that comes later than by one coming earlier.” And we can even extend this list from unicellular eukaryotes (Dunlap et al., 1980) to humans (Aschoff et al., 1975), including contributions to the performance of transmeridian-traveling athletes (Heller et al., 2024). The phenomenon is irrespective of whether 𝝉 is less than or greater than 24 h. One idea is that delays are completed more quickly because they are self-enhancing (Taylor et al., 2021), in the sense that delays expand the time within the circadian cycle for further delay. Intriguingly however, re-entrainment to advancing and delaying temperature cycles in Drosophila appears to be the opposite, with advance shifts proceeding more quickly than delay ones (Abhilash et al., 2020).
Pittendrigh C, Bruce V, Kaus P. On the significance of transients in daily rhythms. Proc Natl Acad Sci USA 44:966-973, 1958 [doi.org/10.1073/pnas.44.9.965].
Dunlap JC, Taylor W, Hastings JW. The effects of protein synthesis inhibitors on the Gonyaulax clock. I. Phase-shifting effects of cycloheximide. J Comp Physiol B 138:1-8, 1980 [doi.org/10.1007/BF00688728].
Aschoff J, Hoffman K, Pohl H, Wever R. Re-entrainment of circadian rhythms after phase-shifts of the zeitgeber. Chronobiologia 2:23-78, 1975 [PMID: 1192905].
Heller HC, Herzog E, Brager A, Poe G, Allada R, Scheer F, Carskadon M, de la Iglesia HO, Jang R, Montero A, Wright K, Mouraine P, Walker MP, Goel N, Hogenesch J, Van Gelder RN, Kriegsfeld L, Mah C, Colwell C, Zeitzer J, Grandner M, Jackson CL, Prichard JR, Kay SA, Paul K. The negative effects of travel on student athletes through sleep and circadian disruption. J Biol Rhythms 39:5-19, 2024 [doi.org/10.1177/07487304231207330].
Taylor S. Delays are self-enhancing: an explanation of the east-west asymmetry in recovery from jetlag. J Biol Rhythms 36:127-136, 2021 [doi.org/10.1177/0748730421990482].
Abhilash L, Kalliyil A, Sheeba V. Responses of activity rhythms to temperature cues evolve in Drosophila populations selected for divergent timing of eclosion. J Exp Biol 223:jeb222414, 2020 [doi.org/10.1242/jeb.222414].
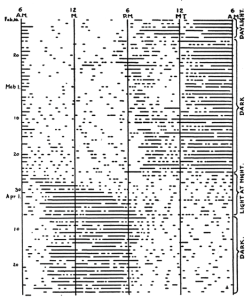
European ground squirrels (Spermophilus citellus) are diurnal, burrow-dwelling rodents that do not see solar light-dark transitions; observational and telemetry data show that the squirrels first emerge from their dark burrows to perceive natural light 3 – 4 h after dawn and retreat back below ground 2 – 3 h before dusk (Hut et al., 1999, and representative actogram below). Nevertheless, their above-ground activity rhythms remain entrained, maintaining a relatively constant phase relationship of activity offset to the changing photoperiod. A plausible entrainment mechanism might involve phase-dependent changes of 𝝉 in response to varying irradiance levels over the duration of the light phase, likely averaged or integrated across several days (Beersma et al., 1999).

Hut RA, van Oort BEH, Daan S. Natural entrainment without dawn and dusk: the case of the European ground squirrel (Spermophilus citellus). J Biol Rhythms 14:290-299, 1999 [doi.org/10.1177/074873099129000704].
Beersma DGM, Daan S, Hut RA. Accuracy of circadian entrainment under fluctuating light conditions: contributions of phase and period responses. J Biol Rhythms 14:320-329, 1999 [doi.org/10.1177/074873099129000740].
It is believed that circadian systems have evolved to enhance fitness, at least in part, by optimizing the timing of internal biological processes to the external day-night cycle. A series of studies has been conducted in cyanobacteria (Synechococcus; Ouyang et al., 1998; Woelfle et al., 2004) and plants (Arabadopsis; Dodd et al., 2005) to address this concept, based on the dependence of entrainment phase on the value of 𝝉 and the period of the entraining light-dark cycle. When short and long 𝝉 mutant bacteria and plants are grown across a range of light-dark cycle lengths, and when short 𝝉 mutant mice are maintained in a semi-natural environment (Spoelstra et al., 2016), those with 𝝉’s similar to the environmental period (referred to as “circadian resonance” by the authors) enjoy competitive advantages over those grown when the two periodicities are mismatched.
Ouyang Y, Andersson CR, Kondo T, Golden SS, Johnson CH. Resonating circadian clocks enhance fitness in cyanobacteria. Proc Natl Acad Sci USA 95:8660-8664, 1998 [doi.org/10.1073/pnas.95.15.8660].
Woelfle MA, Ouyang Y, Phanvijhitsiri K, Johnson CH. The adaptive value of circadian clocks: an experimental assessment in cyanobacteria. Curr Biol 14:1481-1486, 2004 [doi.org/10.1016/j.cub.2004.08.023].
Dodd AN, Salathia N, Hall A, Kévei E, Tóth R, Nagy F, Hibberd JM, Millar AJ, Webb AAR. Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science 309:630-633, 2005 [doi.org/10.1126/science.1115581].
Spoelstra K, Wikelski M, Daan S, Hau M. Natural selection against a circadian clock gene mutation in mice. Proc Natl Acad Sci USA 113:686-691, 2016 [doi.org/10.1073/pnas.1516442113].
In addition to the references in ![]() 2.23 above, see also:
2.23 above, see also:
Aschoff J, Pohl H. Phase relations between a circadian rhythm and its Zeitgeber within the range of entrainment. Naturwissenschaften 65:80-84, 1978 [doi.org/10.1007/BF00440545].
Merrow M, Brunner M, Roenneberg T. Assignment of circadian function for the Neurospora clock gene frequency. Nature 399:584-586, 1999 [doi.org/10.1038/21190].
Duffy JF, Rimmer DW, Czeisler CA. Association of intrinsic circadian period with morningness–eveningness, usual wake time, and circadian phase. Behav Neurosci 115:895-899, 2001 [doi.org/10.1037//0735-7044.115.4.895].
The wheel-running rhythm of golden hamsters offers striking examples to illustrate the large variations of entrainment phase that arise from altered values of 𝝉 or T. The hamster tau mutant is a semi-dominant point mutation in the catalytic region of casein kinase one epsilon (CK1ϵ); the wild-type-typical 𝝉 in constant darkness of about 24 h is shortened to ∽22 h in heterozygotes and ∽20 h in homozygotes. Shown below, the phase of entrained activity onset in a 14 h: 10 h LD cycle occurs normally at the light to dark transition in the wild type (top panel); the heterozygote begins running earlier, during the light phase, several hours before dark onset (middle panel); and the homozygote has essentially become a diurnal rodent (bottom panel).

On the other hand, wild type hamsters can stably entrain their wheel-running rhythms to a 1 h light pulse with a pulse period as short as 23.33 h and as long as 24.67 h, but they align with diametrically opposite phases. In the 23.33 h case, the animal entrains by advances, resulting in its active phase preceding the light pulse (the pulse acts as subjective dawn); while in the 24.67 h case, the animal entrains by delays, resulting in its active phase following the light pulse (the pulse acts as subjective dusk).
Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents. I. The stability and lability of spontaneous frequency. J Comp Physiol 106:223-252, 1976 [doi.org/10.1007/BF01417856].
Schwartz WJ, Tavakoli-Nezhad M, Lambert CM, Weaver DR, de la Iglesia HO. Distinct patterns of Period gene expression in the suprachiasmatic nucleus underlie circadian clock photoentrainment by advances or delays. Proc Natl Acad Sci USA 108:17219-17224, 2011 [doi.org/10.1073/pnas.1107848108].
Here are a few reviews as starting points to enter the literature on photic entrainment of the SCN:
Golombeck DA, Rosenstein RE. Physiology of circadian entrainment. Physiol Rev 90:1063-1102, 2010 [doi.org/10.1152/physrev.00009.2009].
Do MT, Yau KW. Intrinsically photosensitive retinal ganglion cells. Physiol Rev 90:1547-1581, 2010 [doi.org/10.1152/physrev.00013.2010].
Foster RG, Hughes S, Peirson SN. Circadian photoentrainment in mice and humans. Biology 9:180, 2020 [doi.org/10.3390/biology9070180].
Ashton A, Foster RG, Jagannath A. Photic entrainment of the circadian system. Int J Mol Sci 23:729, 2022 [doi.org/10.3390/ijms23020729].
The sensitivity of the human circadian system to light shows wide variation, including across individuals (Phillips et al., 2019), between children and adults (Higuchi et al., 2014), and by eye pigmentation and/or ethnicity (Higuchi et al., 2007).
Phillips AJK, Vidafar P, Burns AC, McGlashan EM, Anderson C, Rajaratnam SMW, Lockley SW, Cain SW. High sensitivity and interindividual variability in the response of the human circadian system to evening light. Proc Natl Acad Sci USA 116:12019-12024, 2019 [doi.org/10.1073/pnas.1901824116].
Higuchi S, Nagafuchi Y, Lee S, Harada T. Influence of light at night on melatonin suppression in children. J Clin Endocrinol Metab 99:3298-3303, 2014 [doi.org/10.1210/jc.2014-1629].
Higuchi S, Motohashi Y, Ishibashi K, Maeda T. Influence of eye colors of Caucasians and Asians on suppression of melatonin secretion by light. Am J Physiol 292:R2352-R2356, 2007 [doi.org/10.1152/ajpregu.00355.2006].
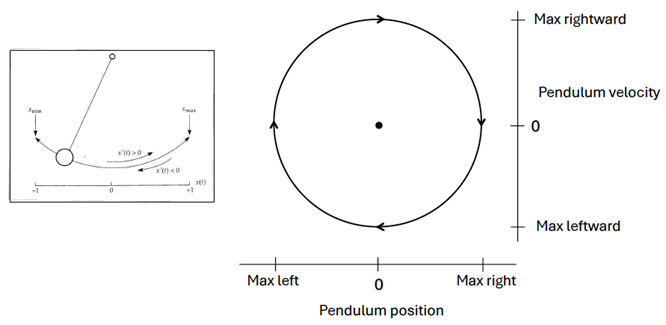
As a graduate student of Pittendrigh, Arthur Winfree (1942 – 2002) pioneered a theoretical model of the self-sustained circadian oscillator as a stable, attractive limit cycle; that is, a closed trajectory in which the state variables of the system exhibit oscillating values. This approach has helped to predict how phase-specific perturbations (like light pulses) affect the oscillator’s behavior. A clock pendulum is often cited as a simple limit cycle defined by two dynamic state variables as the pendulum swings back and forth: its position in space and its velocity. In the phase plane diagram below, you can trace the oscillation as the pendulum moves left and right, with the black dot in the center representing the motionless pendulum, hanging vertically at rest. This equilibrium point is often referred to as a “singularity” or “singular region.”
Perturbing this system at different phases of its oscillation can shift the state variables to other areas of the phase plane. If the strength of the perturbation is weak, such that the shifted system remains on the near side of the singularity, a phase response curve is generated with a continuous transition between small delay and advance phase shifts (Type 1 PRC). On the other hand, if the strength of the perturbation is strong (or the diameter [amplitude] of the limit cycle is small), the shifted system may move to the far side of the singularity, to the opposite side of the limit cycle. This generates a phase response curve with large phase shifts and a discontinuity at the transition between the delays and advances (Type 0 PRC). Illustrated below are generic shapes for Type 1 (left) and Type 0 (right) PRCs to light pulses for a circadian system; the phases of pulse onset (initial circadian phase) are plotted in circadian time and the resulting phase shifts are measured in hours.
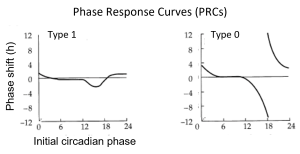
There is another way to display these data, as (PTCs); instead of the phase shifts (in hours) on the y-axis, PTCs plot the new circadian phases (in circadian time) that result from the shifts. Illustrated below are the shapes for Type 1 (left) and Type 0 (right) PTCs that correspond to the PRCs above.
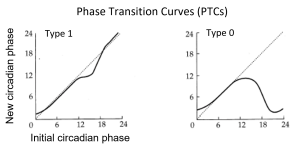
The Type 1 / Type 0 designations for PRCs and PTCs refer to the average slopes of the PTCs, with an average slope of 1 for Type 1 and 0 for Type 0 (dotted lines show a slope of 1). While PRCs have been widely adopted by the circadian field (Johnson, 1999), several topological considerations favor the use of PTCs (Winfree, 2001). Moreover, there is a perceptual advantage to using PTCs rather than PRCs for displaying Type 0 resetting: PTCs avoid the apparent discontinuity at the transition between delays and advances on PRCs. Discontinuous PRCs give the impression that there might be mechanistic differences between the shifts in opposite directions, when in fact the discontinuity is most often an artifact of defining a delay or an advance as no greater than 12 h (one half cycle). Plotting PRCs monotonically – with all phase shifts labeled as delays from 0 h to 24 h – is another approach that can address this confound.
Johnson CH. Forty years of PRCs – what have we learned? Chronobiol Int 16:711-743, 1999 [doi.org/10.3109/07420529909016940].
Winfree AT. The Geometry of Biological Time, second edition, Springer, 2001.
Here we note an interesting notion raised by Winfree’s limit cycle model. Could a light pulse of exactly the right magnitude, delivered at exactly the right phase, lead to circadian arrhythmicity by propelling the oscillator to its singularity, rendering it motionless and phaseless (see arguments in Jewett et al., 1991)? Indeed, attempts to do so with rhythmic cell cultures have led to a loss of rhythmicity, but – given even slight differences in phase between cells or instability of the singular region – these results seem most likely due to desynchronization rather than abolition of individual cellular rhythms (Miwa et al., 1987; see also Nagoshi et al., 2004 and Welsh et al., 2004 for examples of population arrhythmicity due to cellular desynchrony). It’s also important to emphasize that multi-dimensional limit cycles need not be perfect circles with singularities at their centers. For example, a two-dimensional mathematical representation of the Forger/Peskin model oscillator (composed of 73 differential equations) reveals a narrow, elongated oval trajectory with the singularity close to one of its ends (Indic et al., 2006).
Jewett ME, Kronauer RE, Czeisler CA. Light-induced suppression of endogenous circadian amplitude in humans. Nature 350:59-62, 1991 [doi.org/10.1038/350059a0].
Miwa I, Nagatoshi H, Horie T. Circadian rhythmicity within single cells of Paramecium bursaria. J Biol Rhythms 2:57-64, 1987 [doi.org/10.1177/074873048700200105].
Nagoshi E, Saini C, Bauer C, Laroche T, Naef F, Schibler U. Circadian gene expression in individual fibroblasts: cell-autonomous and self-sustained oscillators pass time to daughter cells. Cell 119:693-705, 2004 [doi.org/10.1016/j.cell.2004.11.015].
Welsh DK, Yoo S-H, Liu AC, Takahashi JS, Kay SA. Bioluminescence imaging of individual fibroblasts reveals persistent, independently phased circadian rhythms of clock gene expression. Curr Biol 14:2289-2295, 2004 [doi.org/10.1016/j.cub.2004.11.057].
Indic P, Gurdziel K, Kronauer RE, Klerman EB. Development of a two-dimension manifold to represent high dimension mathematical models of the intracellular mammalian circadian clock. J Biol Rhythms 21:222-232, 2006 [doi.org/10.1177/0748730406287357].
See references:
Spitschan M, Stefani O, Blattner P, Gronfier C, Lockley SW, Lucas RJ. How to report light exposure in human chronobiology and sleep research experiments. Clocks Sleep 1:280-289, 2019 [doi.org/10.3390/clockssleep1030024].
Lucas RJ, Allen AE, Brainard GC, Brown TM, Dauchy RT, Didikoglu A, Do MTH, Gaskill BN, Hattar S, Hawkins P, Hut RA, McDowell RJ, Nelson RJ, Prins J-B, Schmidt TM, Takahashi JS, Verma V, Voikar V, Wells S, Peirson SN. Recommendations for measuring and standardizing light for laboratory mammals to improve welfare and reproducibility in animal research, PLoS Biology 22:e3002535, 2024 [doi.org/10.1371/journal.pbio.3002535].
Lucas RJ, Peirson SN. Practical advice on measuring and applying light for laboratory animals. J Biol Rhythms 39:323-330, 2024 [doi.org/10.1177/07487304241259514].
McDowell RJ, Didikoglu A, Woelders T, Gatt MJ, Moffatt F, Notash S, Hut RA, Brown TM, Lucas RJ. Beyond Lux: Methods for species and photoreceptor-specific quantification of ambient light for mammals. BMC Biology 22:257, 2024 [doi.org/10.1186/s12915-024-02038-1].
Granada et al. (2013) and Bordyugov et al. (2015) have sought a systematic understanding of how entrainment phase relates to 𝝉 – T and Zeitgeber strength using theoretical models and numerical simulations. Their core result is illustrated below.
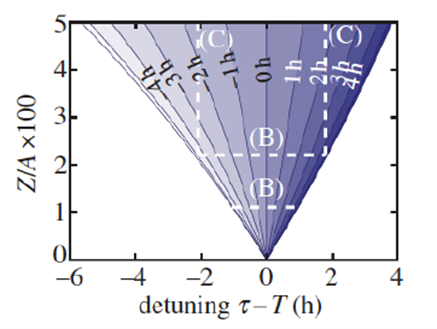
Note that as the ratio of Zeitgeber strength to oscillator amplitude (Z/A) increases (horizontal lines B), the range of entrainment also increases for greater mismatches of 𝝉 – T (within the colored wedge-shaped region termed “Arnold tongue” [after the Russian mathematician V.I. Arnold]). Within the tongue, as the 𝝉 – T mismatch becomes more negative or more positive, the entrainment phase becomes earlier or later, respectively. Vertical lines C show that for a given negative or positive 𝝉 – T mismatch, decreasing Zeitgeber strength leads to an earlier or later entrainment phase, respectively.
Granada AE, Bordyugov G, Kramer A, Herzel H. Human chronotypes from a theoretical perspective. PLoS ONE 8:e59464, 2013 [doi.org/10.1371/journal.pone.0059464].
Winfree realized that the behavior of a population of weakly-coupled limit cycle oscillators would likely be substantially different from that of a single oscillator alone. And he surmised, quite presciently, that intercellular synchronization might determine as much about circadian clock dynamics as does its autonomous cellular mechanism. Indeed, theoretical and experimental studies suggest that the coupling and phase distribution of SCN neurons significantly impact entrainment properties and phase-shifting capacities of the nucleus as a whole (Van der Leest et al., 2009; Abraham et al., 2010).
Van der Leest HT, Rohling JHT, Michel S, Meijer JH. Phase shifting capacity of the circadian pacemaker determined by the SCN neuronal network organization. PLoS ONE 4:e4976, 2009 [doi.org/10.1371/journal.pone.0004976].
Abraham U, Granada AE, Westermark PO, Heine M, Kramer A, Herzel H. Coupling governs entrainment range of circadian clocks. Molec Systems Biol 6:438, 2010 [doi.org/10.1038/msb.2010.92].
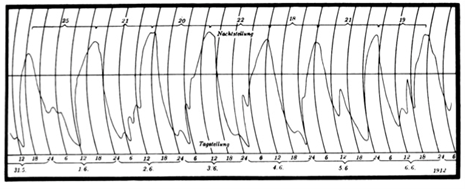
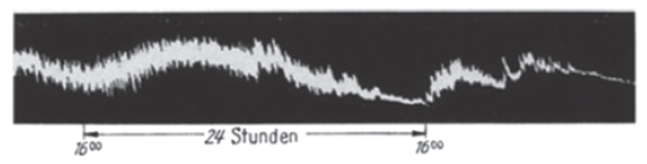
Erwin Bünning (1906 – 1990) (along with Jürgen Aschoff and Colin Pittendrigh, considered one of the three founders of modern circadian biology) noted that some physiological rhythms in vertebrates appear to resynchronize with different speeds after a shift in the light-dark cycle; so inspired, he recorded the peristaltic movements of isolated hamster intestine in a tissue bath over 2-3 days and reported that gut tension expressed a circadian rhythm, with stronger contraction during the projected night. Inspection of his figure (below) suggests why the existence of an intestinal clock was not universally accepted at the time.
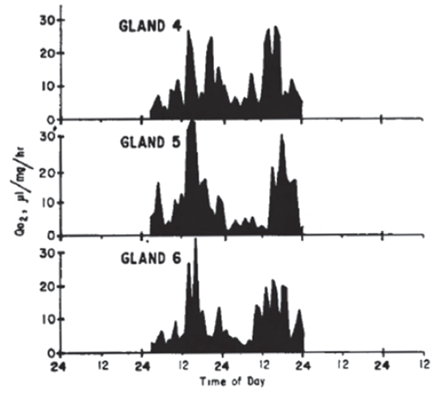
In the 1960’s, Richard Andrews and G. Edgar Folk, Jr. cultured hamster adrenal glands and measured oxygen consumption and steroid secretion over a few days. They made a case for a circadian metabolic rhythm in culture, with low activity during the subjective night, and also described that (a) the phase of the rhythm was not influenced by the time of sacrifice of the donors; (b) the period of the rhythm was relatively independent of flask temperature; and (c) the pattern of the rhythm appeared 12 h out of phase if donor animals were maintained in a reversed light-dark cycle.
And 1 year later, Gerald Tharp and Folk reported a circadian rhythm of heart rate in isolated rat hearts, hypothesizing that “the circadian rhythms of resting heart rate are controlled by a ‘clock’ located within the heart cell.”
Tharp GD, Folk GE Jr. Rhythmic changes in rate of the mammalian heart and heart cells during prolonged isolation. Comp Biochem Physiol 14:255-273, 1965 [doi.org/10.1016/0010-406X(65)90202-1].
The SCN’s efferent pathways are impressively multimodal, translating its electrophysiological rhythm widely through (a) first-order neural projections primarily to nearby hypothalamic areas and then trans-synaptically, including to the autonomic nervous system (with innervation of the pineal gland); and (b) secretion of diffusible substances, with transmission via the ventricular system and a recently-described venous portal system to the organum vasculosum lamina terminalis (OVLT) (Yao et al., 2021). In turn, some of the resulting physiological and behavioral rhythms – of hormone secretion, body temperature, and activity/rest (and consequently, feeding/fasting) – convey external time (or circadian time, in constant conditions) throughout the organism to act as internal Zeitgebers for phase-setting of downstream targets.
Yao Y, Taub AB, LeSauter J, Silver R. Identification of the suprachiasmatic nucleus venous portal system in the mammalian brain. Nat Commun 12:5643, 2021 [doi.org/10.1038/s41467-021-25793-z].
The phase-setting of cell, tissue, and organ rhythms by the SCN involves a multitude of target-specific signaling pathways (for two reviews as starting points, consider Dibner et al., 2010 and Mohawk et al., 2012). The regulation of circadian gene transcription by the signals is primarily, but not exclusively (Kornmann et al., 2007), via the entrainment of local oscillators.
Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: Organization and coordination of central and peripheral clocks. Annu Rev Physiol 72:517-549, 2010 [doi.org/10.1146/annurev-physiol-021909-135821].
Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci 35:445-462, 2012 [doi.org/10.1146/annurev-neuro-060909-153128].
Kornmann B, Schaad O, Bujard H, Takahashi JS, Schibler U. System-driven and oscillator-dependent circadian transcription in mice with a conditionally active liver clock. PLoS Biol 5:e34, 2007 [doi.org/10.1371/journal.pbio.0050034].
On the other hand, the nocturnal rhythm of pineal melatonin synthesis is directly driven via a multi-synaptic neural pathway from the SCN. During the light phase (or subjective day), SCN GABAergic inhibition of the paraventricular nucleus (Kalsbeek et al., 2000) suppresses sympathetic activation of the rate-limiting enzyme for melatonin synthesis, aryl alkylamine N-acetyltransferase (AANAT). During the dark phase (or subjective night), the inhibition is released, and along with a stimulatory signal from a small group of glutamatergic SCN neurons (Perreau-Lenz et al., 2004), melatonin synthesis can proceed. Incidentally, in addition to its other roles (see Chapter 3), the melatonin rhythm acts as an internal Zeitgeber during pregnancy; it is sufficient (although not necessary) as a transplacental entraining signal of the fetal circadian system (Davis and Mannion, 1988).
Kalsbeek A, Garidou ML, Palm IF, Van Der Vliet J, Simonneaux V, Pévet P, Buijs RM. Melatonin sees the light: blocking GABA-ergic transmission in the paraventricular nucleus induces daytime secretion of melatonin. Eur J Neurosci 12:3146-3154, 2000
[doi.org/ /10.1046/j.1460-9568.2000.00202.x].
Perreau-Lenz, Kalsbeek A, Pévet P, Buijs RM. Glutamatergic clock output stimulates melatonin synthesis at night. Eur J Neurosci 19:318-324, 2004 [doi.org/10.1111/j.0953-816x.2003.03132.x].
Davis FC, Mannion J. Entrainment of hamster pup circadian rhythms by prenatal melatonin injections to the mother. Am J Physiol 255:R439-R448, 1988 [doi.org/10.1152/ajpregu.1988.255.3.R439].
Yet another mechanism appears responsible for the SCN’s control of the locomotor activity rhythm in nocturnal rodents. Rather than entraining or driving the rhythm, the SCN acts to “gate” locomotor activity by allowing expression during the dark phase (or subjective night) and suppressing it during the light phase (or subjective day), when the rhythm of overall SCN electrical activity is low or high, respectively. The width of this permissive window is defined by SCN activity falling below or rising above a 50% level (Houben et al., 2009; see also Davis and Menaker, 1980). Note that this tight relationship can be altered under certain circumstances (see ![]() 2.43 in this chapter and
2.43 in this chapter and ![]() 3.2 in Chapter 3).
3.2 in Chapter 3).
Houben T, Deboer T, van Oosterhout F, Meijer JH. Correlation with behavioral activity and rest implies circadian regulation by SCN neuronal activity levels. J Biol Rhythms 24:477–487, 2009 [doi.org/10.1177/0748730409349895].
Davis FC, Menaker M. Hamsters through time’s window: temporal structure of hamster locomotor rhythmicity. Am J Physiol 239:R149-R155, 1980 [doi.org/10.1152/ajpregu.1980.239.1.R149].
With the advent and advances in genome-wide transcriptomic and proteomic studies came the realization that not all rhythmic transcripts lead to protein rhythms and not all protein rhythms are encoded by rhythmic transcripts. Of course, rates of both production and degradation determine the levels of mRNA and protein abundance; models suggest that, depending on the mean half-lives of the molecules, rhythmic degradation itself can shape the amplitudes and peak phases of the molecular rhythms (Lück et al., 2014; Wang et al., 2018). In any case, a practical lesson is that the presence or absence of rhythmic transcripts alone (or their amplitudes and phases) cannot define the functional rhythmicity of circadian systems.
Lück S, Thurley K, Thaben PF, Westermark PO. Rhythmic degradation explains and unifies circadian transcriptome and proteome data. Cell Rep 9:741-751, 2014 [doi.org/10.1016/j.celrep.2014.09.021].
Wang J, Symul L, Yeung J, Gobet C, Sobel J, Lück S, Westermark PO, Molina N, Naef F. Circadian clock-dependent and -independent posttranscriptional regulation underlies temporal mRNA accumulation in mouse liver. Proc Natl Acad Sci USA 115:E1916-E1925, 2018 [doi.org/10.1073/pnas.1715225115].
In addition to critical roles in photic entrainment (see ![]() 2.31 in this chapter) and seasonal encoding (see Chapter 3), cellular communication within the SCN – established by complex mechanisms of intercellular coupling and topographical compartmentalization – is key for building an SCN pacemaker that is precise (Herzog et al., 2004), robust (Liu et al., 2007), and resistant to entrainment by the body temperature rhythm (Buhr et al., 2010). For reviews of candidate mechanisms, see Evans and Gorman, 2016 and Herzog et al., 2017.
2.31 in this chapter) and seasonal encoding (see Chapter 3), cellular communication within the SCN – established by complex mechanisms of intercellular coupling and topographical compartmentalization – is key for building an SCN pacemaker that is precise (Herzog et al., 2004), robust (Liu et al., 2007), and resistant to entrainment by the body temperature rhythm (Buhr et al., 2010). For reviews of candidate mechanisms, see Evans and Gorman, 2016 and Herzog et al., 2017.
Herzog ED, Aton SJ, Numano R, Sakaki Y, Tei H. Temporal precision in the mammalian circadian system: a reliable clock from less reliable neurons. J Biol Rhythms 19:35-46, 2004 [doi.org/10.1177/0748730403260776].
Liu AC, Welsh DK, Ko CH, Tran HG, Zhang EE, Priest AA, Buhr ED, Singer O, Meeker K, Verma IM, Doyle FJ III, Takahashi JS, Kay SA. Intercellular coupling confers robustness against mutations in the SCN circadian clock network. Cell 129:605-616, 2007 [doi.org/10.1016/j.cell.2007.02.047].
Buhr ED, Yoo S-H, Takahashi JS. Temperature as a universal resetting cue for mammalian circadian oscillators. Science 330:379-385, 2010 [doi.org/10.1126/science.1195262].
Evans JA, Gorman MR. In synch but not in step: circadian clock circuits regulating plasticity in daily rhythms. Neuroscience 320:259-280, 2016 [doi.org/10.1016/j.neuroscience.2016.01.072].
Herzog ED, Hermanstyne T, Smyllie NJ, Hastings MH. Regulating the suprachiasmatic nucleus (SCN) circadian clockwork: interplay between cell-autonomous and circuit-level mechanisms. Cold Spring Harb Perspect Biol 9:a027706, 2017 [doi.org/10.1101/cshperspect.a027706].
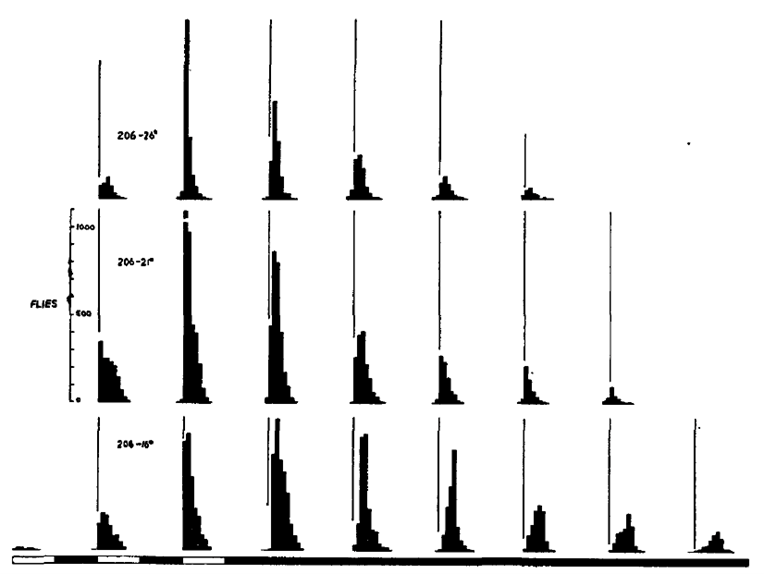
Originally described as temperature independence, Pittendrigh argued that such a property must be required for a physiological clock to function as a reliable timekeeper and not as a thermometer (Pittendrigh, 1960). He made the case with a classical set of experiments on the timing of Drosophila eclosion under different ambient temperatures (Pitttendrigh, 1954), also making a point that the internal circadian timekeeping mechanism is separate from the downstream behaviors it regulates (see also ![]() 2.6 in this chapter).
2.6 in this chapter).
Pittendrigh CS. Circadian rhythms and the circadian organization of living systems. Cold Spring Harb Symp Quant Biol 25:159-184, 1960 [doi.org/10.1101/sqb.1960.025.01.015].
Pittendrigh CS. On temperature independence in the clock system controlling emergence time in Drosophila. Proc Natl Acad Sci USA 40:1018-1029, 1954 [doi.org/10.1073/pnas.40.10.1018].
The relative roles of the retina, pineal, and SCN in the circadian systems of fish, reptiles, birds, and mammals roughly correspond along phylogenetic lines (but important details are variable even within a vertebrate class). Differing roles for the structures might also reflect evolutionary adaptation to different photic niches and also changing requirements of developmental life stages (Menaker et al., 1997). Now thanks to the availability of modern genome editing technologies, a revitalized comparative approach incorporating novel animal models can take advantage of the strengths of additional species.
Menaker M, Moreira LF, Tosini G. Evolution of circadian organization in vertebrates. Braz J Med Biol Res 30:305-313, 1997 [doi.org/10.1590/S0100-879X1997000300003].
We have already pointed out that the rodent running wheel is not only an assay of the locomotor activity rhythm but also a modulator of it (see ![]() 2.7). The mechanisms appear complex – involving effects related to exercise and central reward pathways – but also include “feedback” on the electrophysiological activity of the SCN itself, enhancing the amplitude of its circadian electrical rhythm (Van Oosterhout et al., 2012; Houben et al., 2014). In this regard, it’s interesting that provision of a running wheel to the common vole (Microtus arvalis) enhances the circadian component of its activity rhythm but suppresses the ultradian component of its activity/rest and feeding/fasting rhythms, especially during the light phase. Although electrical activity has not been recorded in the vole SCN, clock gene expression in the vole nucleus remains circadian in the presence or absence of the wheel (Van der Veen et al., 2006).
2.7). The mechanisms appear complex – involving effects related to exercise and central reward pathways – but also include “feedback” on the electrophysiological activity of the SCN itself, enhancing the amplitude of its circadian electrical rhythm (Van Oosterhout et al., 2012; Houben et al., 2014). In this regard, it’s interesting that provision of a running wheel to the common vole (Microtus arvalis) enhances the circadian component of its activity rhythm but suppresses the ultradian component of its activity/rest and feeding/fasting rhythms, especially during the light phase. Although electrical activity has not been recorded in the vole SCN, clock gene expression in the vole nucleus remains circadian in the presence or absence of the wheel (Van der Veen et al., 2006).
Much of the past work on SCN neural inputs initially focused on innervation by the retina (retinohypothalamic tract), intergeniculate leaflet (geniculohypothalamic tract), and median raphe nucleus. But the SCN receives on the order of hundreds of monosynaptic and polysynaptic non-photic inputs from interoceptive neural systems, including olfactory, limbic, circumventricular, hypothalamic, visceral, and nociceptive sites (Krout et al., 2002). And the SCN bears high-affinity receptors for melatonin (Dubocovich, 2007) and sex hormones (Karatsoreos et al., 2007; Hatcher et al., 2020) and responds to humoral factors that regulate energy metabolism (see Bass, 2013) and inflammation (Cermakian et al., 2014).
Van Oosterhout F, Lucassen EA, Houben T, van der Leest HT, Antle MC, Meijer JH. Amplitude of the SCN clock enhanced by the behavioral activity rhythm. PLoS ONE 7:e39693, 2012 [doi.org/10.1371/journal.pone.0039693].
Houben T, Coomans CP, Meijer JH. Regulation of circadian and acute activity levels by the murine suprachiasmatic nuclei. PLoS ONE 9:e110172, 2014 [doi.org/10.1371/journal.pone.0110172].
Van der Veen FR, Le Minh N, Gos P, Americ M, Gerkema MP, Schibler U. Impact of behavior on central and peripheral circadian clocks in the common vole Microtus arvalis, a mammal with ultradian rhythms. Proc Natl Acad Sci USA 103:3393-3398, 2006 [doi.org/10.1073/pnas.0507825103].
Krout KE, Kawano J, Mettenleiter TC, Loewy AD. CNS inputs to the suprachiasmatic nucleus of the rat. Neuroscience 110:73-92, 2002 [doi.org/10.1016/s0306-4522(01)00551-6].
Dubocovich ML. Melatonin receptors: role on sleep and circadian rhythm regulation. Sleep Med 8:S34-S42, 2007 [doi.org/10.1016/j.sleep.2007.10.007].
Karatsoreos IN, Wang A, Sasanian J, Silver R. A role for androgens in regulating circadian behavior and the suprachiasmatic nucleus. Endocrinology 148:5487-5495, 2007 [doi.org/10.1210/en.2007-0775].
Hatcher KM, Royston SE, Mahoney MM. Modulation of circadian rhythms through estrogen receptor signaling. Eur J Neurosci 51:217-228, 2020 [doi.org/10.1111/ejn.14184].
Bass J. Forever (FGF) 21. Nat Med 19:1090-1092, 2013 [doi.org/10.1038/nm.3334].
Cermakian N, Westfall S, Kiessling S. Circadian clocks and inflammation: reciprocal regulation and shared mediators. Arch Immunol Ther Exp 62:303-318, 2014 [doi.org/10.1007/s00005-014-0286-x].
A prime example of the SCN’s position at the nexus of a circadian and homeostatic pathway is in the neural regulation of thirst. The murine SCN drives anticipatory thirst, secretion of anti-diuretic hormone, and a reduction in body temperature a few hours before sleep to protect against dehydration. Projections from SCN vasopressin neurons to the OVLT are necessary and sufficient to drive circadian thirst (Gizowski and Bourque, 2018). But the homeostatic response to an unanticipated rise in serum osmolality (hypertonic saline injection, ZT 19) is also mediated by SCN vasopressin neurons, driven by an excitatory GABA-ergic input from osmo-sodium-sensing neurons in the OVLT (Gizowski and Bourque, 2020).
Gizowski C, Bourque CW. The neural basis of homeostatic and anticipatory thirst. Nat Rev Nephrol 14:11-25, 2018 [doi.org/10.1038/nrneph.2017.149].
Gizowski C, Bourque CW. Sodium regulates clock time and output via an excitatory GABAergic pathway. Nature 583:421-424, 2020 [doi.org/10.1038/s41586-020-2471-x].
A rapidly expanding list of communicating signals, cellular mediators, and clock gene targets are being identified with local actions that bridge oscillatory functions across neighboring and distant tissues and organs. For two reviews as starting points:
Sinturel F, Petrenko V, Dibner C. Circadian clocks make metabolism run. J Molec Biol 432:3680-3699, 2020 [doi.org/10.1016/j.jmb.2020.01.018].
Koronowski KB, Sassone-Corsi P. Communicating clocks shape circadian homeostasis. Science 371:eabd0951, 2021 [doi.org/10.1126/science.abd0951].
Local oscillators regulate aspects of circadian gene expression in cornea and retina, but their entrainment to the light-dark cycle does not require the SCN, melanopsin (OPN4), or rod or cone opsins. Instead, neuropsin (OPN5) – already known as a deep brain photopigment in birds – has been identified as the responsible opsin, expressed in a small subset of retinal ganglion cells (Buhr et al., 2015). Neuropsin is also present in murine melanocytes – as in some invertebrates and non-mammalian vertebrates – and can mediate photic entrainment of local epidermal oscillators (Buhr et al., 2019). Additional possible SCN-independent actions of light on the coherence of circadian network elements are not fully understood (Husse et al., 2014; Koronowski et al., 2019; Welz et al., 2019).
Buhr ED, Yue WWS, Ren X, Jiang Z, Liao H-WR, Mei X, Vemaraju S, Nguyen M-T, Reed RR, Lang RA, Yau K-W, Van Gelder RN. Neuropsin (OPN5)-mediated photoentrainment of local circadian oscillators in mammalian retina and cornea. Proc Natl Acad Sci USA 112:13093-13098, 2015 [doi.org/10.1073/pnas.1516259112].
Buhr ED, Vemaraju S, Diaz N, Lang RA, Van Gelder RN. Neuropsin (OPN5) mediates local light-dependent induction of circadian clock genes and circadian photoentrainment in exposed murine skin. Curr Biol 29:3478-3487, 2019 [doi.org/10.1016/j.cub.2019.08.063].
Husse J, Leliavski A, Tsang AH, Oster H, Eichele G. The light-dark cycle controls peripheral rhythmicity in mice with a genetically ablated suprachiasmatic nucleus clock. FASEB J 28:4950-4960, 2014 [doi.org/10.1096/fj.14-256594].
Koronowski KB, Kinouchi K, Welz P-S, Smith JG, Zinna VM, Shi J, Samad M, Chen S, Magnan CN, Kinchen JM, Li W, Baldi P, Benitah SA, Sassone-Corsi P. Defining the independence of the liver circadian clock. Cell 177:1448-1462, 2019 [doi.org/10.1016/j.cell.2019.04.025].
Welz P-S, Zinna VM, Symeonidi A, Koronowski KB, Kinouchi K, Smith JG, Guillén IM, Castellanos A, Furrow S, Aragón F, Crainiciuc G, Prats N, Caballero JM, Hidalgo A, Sassone-Corsi P, Benitah SA. BMAL1-drive tissue clocks respond independently to light to maintain homeostasis. Cell 177:1436-1447, 2019 [doi.org/10.1016/j.cell.2019.05.009].
An example of a developmental realignment of oscillating network components is in developing rats, which show a nearly 12-h shift of the liver and thyroid oscillators’ phase with respect to that of the SCN around post-natal days 20 – 22, corresponding to the switch from suckling during mother’s rest phase (light phase) to independent feeding during the pups’ own active phase (dark phase) (Yamazaki et al., 2009). And in laboratory mice, increasing the energetic costs of feeding – rather than displacing and restricting its timing – or lowering ambient temperature can functionally reconfigure the network phenotype from nocturnal to essentially diurnal (Van der Vinne et al., 2014; we will consider the likely adaptive significance of such “temporal niche” switching in Chapter 3).
Yamazaki S, Yoshikawa T, Biscoe EW, Numano R, Gallaspy LM, Soulsby S, Papadimas E, Pezuk P, Doyle SE, Tei H, Sakaki Y, Block GD, Menaker M. Ontogeny of circadian organization in the rat. J Biol Rhythms 24:55-63, 2009 [doi.org/10.1177/0748730408328438].
Van der Vinne V, Riede SJ, Gorter JA, Eijer WG, Sellix MT, Menaker M, Daan S, Pilorz V, Hut RA. Cold and hunger induce diurnality in a nocturnal mammal. Proc Natl Acad Sci USA 111:15256-15260, 2014 [doi.org/10.1073/pnas.1413135111].
Increasingly (and well beyond our forced switches between standard and daylight savings times), human activity is affecting the daily timing of other species, either directly by invasion of their ecosystems or indirectly through climate change and light pollution. The consequences – both adaptive and maladaptive – have only begun to be explored; for a review and research agenda see:
Gilbert NA, McGinn KA, Nunes LA, Shipley AA, Bernath-Plaisted J, Clare JDJ, Murphy PW, Keyser SR, Thompson KL, Maresh Nelson SB, Cohen JM, Widick IV, Bartel SL, Orrock JL, Zuckerberg B. Daily activity timing in the Anthropocene. Trends Ecol Evol 38:324-336, 2023 [doi.org/10.1016/j.tree.2022.10.008].
Charles Ehret (1923 – 2007) coined the term chronotype as the overall temporal phenotype of an organism; for example, he illustrated the circadian chronotype of the rat in a 12 h: 12 h light-dark cycle from a review of the literature:
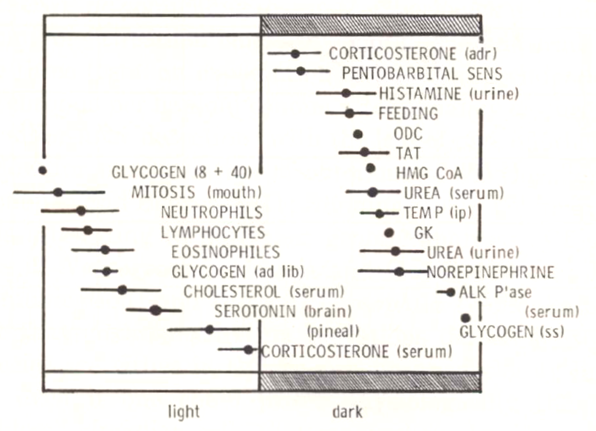
Presciently, in the paper cited above, Ehret quoted a 1972 student paper in “circadian cybernetics” (T. Hefty, University of Wisconsin, Madison) that “even if the molecular circadian clock is someday well defined, working out the complexities of the interactions of these ‘little clocks’ to form larger systems will still be a monumental task.”
See references:
Kasukawa T, Sugimoto M, Hida A, Minami Y, Mori M, Honma S, Honma K, Mishima K, Soga T, Ueda HR. Human blood metabolite timetable indicates internal body time. Proc Natl Acad Sci USA 109:15036–15041, 2012 [doi.org/10.1073/pnas.1207768109].
Hughey JJ. Machine learning identifies a compact gene set for monitoring the circadian clock in human blood. Genome Med 9:19, 2017 [doi.org/10.1186/s13073-017-0406-4].
Laing EE, Möller-Levet CS, Poh N, Santhi N, Archer SN, Dijk DJ. Blood transcriptome based biomarkers for human circadian phase. eLife 6:e20214, 2017 [doi.org/10.7554/eLife.20214].
Wittenbrink N, Ananthasubramaniam B, Münch M, Koller B, Maier B, Weschke C, Bes F, de Zeeuw J, Nowozin C, Wahnschaffe A, Wisniewski S, Zaleska M, Bartok O, Ashwal-Fluss R, Lammert H, Herzel H, Hummel M, Kadener S, Kunz D, Kramer A. High-accuracy determination of internal circadian time from a single blood sample. J Clin Invest 128:3826-3839, 2018 [doi.org/10.1172/JCI120874]
Huang Y, Braun R. Platform-independent estimation of human physiological time from single blood samples. Proc Natl Acad Sci USA 121:e2308114120, 2024 [doi.org/10.1073/pnas.2308114120].
Here are a few reviews as starting points to enter the burgeoning literature on the association of circadian dysrhythmias and the possible etiopathogenesis of neuropsychiatric, cardiovascular, metabolic, neoplastic, and immune disorders:
Fishbein AB, Knutson KL, Zee PC. Circadian disruption and human health. J Clin Invest 131:e148286, 2021 [doi.org/10.1172/JCI148286].
Colwell CS. Defining circadian disruption in neurodegenerative disorders. J Clin Invest 131:e148288, 2021 [doi.org/10.1172/JCI148288].
Fekry B, Eckel-Mahan K. The circadian clock and cancer: links between circadian disruption and disease pathology. J Biochem 171:477-486, 2022 [doi.org/10.1093/jb/mvac017].
Schrader LA, Ronnekleiv-Kelly SM, Hogenesch JB, Bradfield CA, Malecki KMC. Circadian disruption, clock genes, and metabolic health. J Clin Invest 134:e170998, 2024 [doi.org/10.1172/JCI170998].
Electroencephalographic slow wave activity (SWA, oscillations in the delta band 0.5 – 4 Hz) is believed to reflect the homeostatic sleep regulatory process (“sleep need”), as SWA increases with the duration of wakefulness and decreases with daytime naps and over the course of sleep (Borbély, 1982; Daan et al., 1984; Dijk et al., 1990, 1995; Werth et al., 1996). The rise and fall of the process during episodes of sleep deprivation has been mathematically modeled (see Fig. 17); advanced analyses are complicated by variation in local SWA time courses across cortical regions and with chronic sleep restriction over multiple nights.
For a review of the two process (circadian + homeostatic) model of sleep regulation, see:
Borbély A. The two-process model of sleep regulation: Beginnings and outlook. J Sleep Res 31:e13598, 2022 [doi.org/10.1111/jsr.13598].
Borbély AA. A two process model of sleep regulation. Human Neurobiol 1:195–204, 1982 [PMID: 7185792].
Daan S, Beersma DG, Borbély A. Timing of human sleep: recovery process gated by a circadian pacemaker. Am J Physiol 246:R161-183, 1984 [doi.org/10.1152/ajpregu.1984.246.2.R161].
Dijk D-J, Brunner DP, Borbély A. Time course of EEG power density during long sleep in humans. Am J Physiol 258:R650-661, 1990 [doi.org/10.1152/ajpregu.1990.258.3.R650].
Dijk D-J. EEG slow waves and sleep spindles: windows on the sleeping brain. Behav Brain Res 69:109-116, 1995 [doi.org/10.1016/0166-4328(95)00007-g].
Werth E, Dijk D-J, Achermann P, Borbély AA. Dynamics of the sleep EEG after an early evening nap: experimental data and simulations. Am J Physiol 271:R501-510, 1996 [doi.org/10.1152/ajpregu.1996.271.3.R501].
The sleep-wake circadian rhythm of C57BL/6 male mice is altered by the provision of a running wheel; with the wheel locked, total wake time decreases and non-REM and REM sleep time increases by about 11%, without a change in the amplitude of the drinking rhythm. However, the 𝝉-shortening effect of running in the wheel (see ![]() 2.7 in this chapter) impacts both sleep-wake and drinking rhythms equally.
2.7 in this chapter) impacts both sleep-wake and drinking rhythms equally.
Edgar DM, Kilduff TS, Martin CE, Dement WC. Influence of running wheel activity on free-running sleep-wake and drinking circadian rhythms in mice. Physiol Behav 50:373-378, 1991 [doi.org/10.1016/0031-9384(91)90080-8].
See:
Daan S, Hut RA. Circadian clock, program, oscillator, pacemaker, synchroniser?: On the language of biological rhythms research. In: Circadian Clocks (Honma K, Honma S, eds), Hokkaido University Press, 2016, pp. 21-32.
Aviram R, Manella G. A metaphor that keeps on ticking: the “clock” as a driving force in the history of chronobiology research. Philos Theor Pract Biol 12:7, 2020 [doi.org/10.3998/ptpbio.16039257.0012.006].
Even before the hereditary basis for the circadian clock was demonstrated, its inborn nature had already been realized; and, of course, the study of leaf movements led the way. Richard Semon (1859 – 1918), who went on to consider the nature of memories (he coined the term “engram”), published data on young beansprouts (Phaseolus) that had never seen any light, were then exposed to light-dark cycles of 12, 24 or 48 h, and afterwards returned to constant conditions. The plants expressed only a circa 24-h period (Semon, 1905). And in 1928, the Dutch botanist Anthonia Kleinhoonte (1887 – 1960) published her work on jack bean plants (Canavalia ensiformis), demonstrating that leaf movements exhibited a circa-24 h rhythm in constant darkness even if the seedlings were first maintained in an 8 h: 8 h light-dark cycle (Kleinhoonte, 1928). She also reported that brief light pulses – as short as one minute in duration – could reset rhythm phase depending on the phase of administration of the light!
Semon R. Über die Erblichkeit der Tagesperiode. Biologisches Centralblatt 25:241-252, 1905.
Kleinhoonte A. De door het licht geregelde autonome bewegingen der Canavalia-bladeren. Meinema, Delft, 1928.
Some microtine rodents are nocturnal in summer and diurnal in winter, and this shift can be induced in the laboratory by changing photoperiod alone (for example, in the montane vole [Microtus montanus]; Rowsemitt et al., 1982). Such temporal niche switching has been proposed as a seasonal strategy for adapting to energetic challenges (e.g., cold, hunger), and the mechanism appears to lie downstream of the SCN, as assayed by the unchanging phase of SCN clock gene expression (Van der Vinne et al., 2015). Conservation of SCN phase is also a feature of animals that are naturally diurnal or nocturnal in their locomotor activity rhythms or that switch their rhythm phase when provided with a running wheel (for review, see Smale et al., 2003).
Rowsemitt CN, Petterborg LJ, Claypool LE, Hoppensteadt FC, Negus NC, Berge PJ. Photoperiodic induction of diurnal locomotor activity in Microtus montanus, the montane vole. Can J Zool 60:2798-2803, 1982 [doi.org/10.1139/z82-358].
Van der Vinne V, Riede SJ, Gorter JA, Eijer WG, Sellix MT, Menaker M, Daan S, Pilorz V, Hut RA. Cold and hunger induce diurnality in a nocturnal mammal. Proc Natl Acad Sci USA 111:15256-15260, 2014 [doi.org/10.1073/pnas.1413135111].
Smale L, Lee T, Nunez AA. Mammalian diurnality: some facts and gaps. J Biol Rhythms 18:356-366, 2003 [doi.org/10.1177/0748730403256651].
The critical photoperiod is the light phase duration required for a biological response to occur (e.g., flowering in plants, reproductive status in animals) and is influenced by a number of factors. For example, horses are certainly large mammals, but breeding occurs mostly in spring rather than autumn, given their very long gestation time of about 11 months (Bos and Van der Mey, 1980). Differences in the steepness of a population’s photoperiodic response curve – the slope at the inflection point – may reflect individual variation in photoperiodic sensitivity. A shallower curve suggests that there may be a role for other environmental features that are less predictable from year to year, like food availability and ambient temperature, to shape the photoperiodic response to optimally time seasonal responses (Van Rosmalen and Hut, 2021). Of note, annual rhythms of both light and temperature vary with differences in latitude, and critical photoperiod within a species is known to vary with distance from the equator (for review, see Hut et al., 2013). Importantly, the critical photoperiod must also depend on photoperiodic history. In nature, each daylength (except at the solstices) occurs twice per year and thus could signal either upcoming winter or upcoming summer, depending on the trajectory of preceding daylengths (Gorman and Zucker, 1995; Butler et al., 2007).
Bos H, Van der Mey GJW. Length of gestation periods of horses and ponies belonging to different breeds. Livestock Prod Sci 7:181-187, 1980 [doi.org/10.1016/0301-6226(80)90105-0].
Van Rosmalen L, Hut RA. Food and temperature change photoperiodic responses in two vole species. J Exp Biol 224:jeb243030, 2021 [doi.org/10.1242/jeb.243030].
Hut RA, Paolucci S, Dor R, Kyriacou CP, Daan S. Latitudinal clines: an evolutionary view on biological rhythms. Proc Biol Sci 280:20130433, 2013 [doi.org/10.1098/rspb.2013.0433].
Gorman MR, Zucker I. Seasonal adaptations of Siberian hamsters. II. Pattern of change in daylength controls annual testicular and body weight rhythms. Biol Reprod 53:116-125, 1995 [doi.org/10.1095/biolreprod53.1.116].
Butler MP, Turner KW, Park JH, Butler JP, Trumbull JJ, Dunn SP, Villa P, Zucker I. Simulated natural day lengths synchronize seasonal rhythms of asynchronously born male Siberian hamsters. Am J Physiol 293:R402-R412, 2007 [doi.org/10.1152/ajpregu.00146.2007].
Short-lived seasonal species (for example, the Siberian [Djungarian] hamster [Phodopus sungorus]) generally don’t survive for more than one breeding season, so laboratory research has focused on their reproductive activation with the lengthening of the photoperiod that naturally occurs from winter to spring. However, even if the animals are maintained on the short (simulated winter) photoperiod, reproductive activation will still occur after an interval of weeks to months (that is, the animals become refractory to the short-photoperiod inhibitory condition). Once they become reproductively active, though, they do not undergo any further changes in their physiological state. On the other hand, long-lived animals that breed repeatedly over two or more years will undergo continued circannual cycles when maintained on a daily light-dark cycle of a fixed photoperiod.
For reviews of the properties of photoperiodic and circannual timing schemes, see:
Paul MJ, Zucker I, Schwartz WJ. Tracking the seasons: the internal calendars of vertebrates. Phil Trans R Soc B 363:341-361, 2008 [doi.org/10.1098/rstb.2007.2143].
Lincoln G. A brief history of circannual time. J Neuroendocrinol 31:e12694, 2019 [doi.org/10.1111/jne.12694].
It was Bünning (1936) who put forward the hypothesis that photoperiodic (seasonal) time measurement might involve an underlying circadian mechanism. Over the next decades supportive evidence was gathered, based largely on two experimental protocols that were initially tested in seasonally-flowering plants. Using a short photoperiod with an extended dark phase that was interrupted by brief light pulses delivered at different times, Robert C. Bünsow (1953) and others could inhibit or stimulate the flowering of short-day or long-day plants, respectively, if the light pulses were delivered 24 h apart.
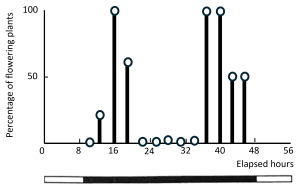
Drawn from the original data in: Claes H, Lang A. Die Blütenbildung von Hyoscyamus niger in 48-stündigen Licht-Dunkel-Zyklen und in Zyklen mit aufgeteilten Lichtphasen. Z Naturforsch 2b:56–63, 1947 [doi.org/10.1515/znb-1947-1-214]; under Creative Commons Attribution License 3.0. See the data also graphed as Fig. 100 in: Bünning E. Die Physiologische Uhr. Springer-Verlag, Berlin, 1958.
Karl C. Hamner and his students, most prolifically with K.K. Nanda (Nanda and Hamner, 1958), designed a “resonance” protocol, with light-dark cycles ranging from 18 to 72 h, all typically with an 8 h light phase but extended dark phases. Under these conditions, flowering of a short-day plant could be stimulated if the period of the light-dark cycle (T) was a multiple of 24 h (see example below).
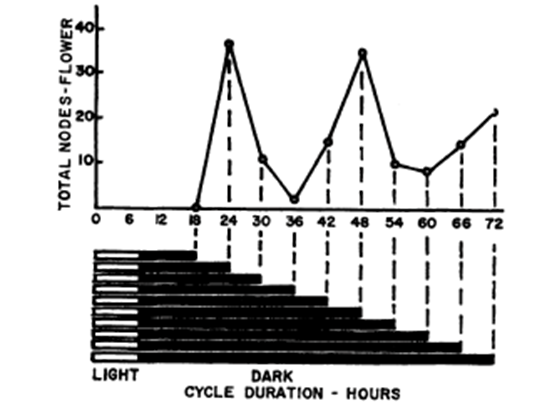
By permission, from Fig. 1 in: Hamner KC. Photoperiodism and circadian rhythms. Cold Spring Harb Symp Quant Biol 25:269-277, 1960 [doi.org/10.1101/SQB.1960.025.01.029].
Following these early studies, Bünsow and Nanda-Hamner protocols have since been applied to assess the circadian contribution to seasonal adaptation in a number of animals, especially for the regulation of winter diapause in insects (see Meuti and Denlinger, 2013).
Bünning E. Die endonome Tagershythmik als Grundlage der photoperiodischen Reaktion. Ber Deut Bot Ges 54:590-607, 1936 [doi.org/10.1111/j.1438-8677.1937.tb01941.x].
Bünsow RC. Über Tages- und Jahresrhythmische Anderugen der Photoperiodischen Lichteropfindlischkeit bie Kalanchoe blossfeldiana und ihre Bezieghugen zuer endogogen Tagesrhythmik. Z Bot 41:257-276, 1953.
Nanda KK, Hamner KC. Studies on the nature of the endogenous rhythm affecting photoperiodic response of Biloxi soybean. Bot Gaz 120:14-25, 1958 [doi.org/10.1086/335992].
Meuti ME, Denlinger DL. Evolutionary links between circadian clocks and photoperiodic diapause in insects. Integr Comp Biol 53:131-143, 2013 [doi.org/10.1093/icb/ict023].
The intrinsic SCN oscillation that is most obviously altered by ambient photoperiod is its rhythmic waveform. Under long photoperiods, the light phase and subjective day are characterized by expanded durations of elevated electrical activity; under short photoperiods, the durations are compressed. The underlying basis for the plasticity in overall waveform has been clarified by analyses of murine SCN rhythms at the cellular level, by recording electrical activity and imaging bioluminescent clock gene reporter expression. The data reveal that photoperiod is encoded by a spatiotemporal reconfiguration of the phases of individual oscillating SCN cells – dispersed dorsoventrally or rostro-caudally during long photoperiods and more narrowly during short photoperiods – rather than by an expansion or compression of the rhythm duration of individual cells (for discussion and references, see Evans and Schwartz, 2024). In Drosophila too, discrete groups of circadian pacemaker neurons generate Ca2+ rhythms with altered phases under long and short photoperiods (Liang et al., 2016).
Evans JA, Schwartz WJ. On the origin and evolution of the dual oscillator model underlying the photoperiodic clockwork in the suprachiasmatic nucleus. J Comp Physiol A 210: 503-511, 2024 [doi.org/10.1007/s00359-023-01659-1].
Liang X, Holy TE, Taghert PH. Synchronous Drosophila circadian pacemakers display non-synchronous Ca2+ rhythms in vivo. Science 351:976-981, 2016 [doi.org/10.1126/science.aad3997].
In Soay sheep (Dardente et al., 2010) and melatonin-proficient CBA/N mice (Masumoto et al., 2010), a molecular switch governing pars tuberalis TSHβ transcription has been described. Under long photoperiods, the melatonin signal engages the local circadian oscillator in the pars tuberalis, inducing the expression of coactivator eyes absent 3 (eya3); Eya3 interacts with the circadian transcription factor thyrotroph embryonic factor (TEF) on the D box response element of the TSHβ promoter to activate TSHβ transcription in pars tuberalis cells. Notably, Eya3 forms a positive feedback by reinforcing its own transcription, thereby creating a molecular switch that augments transcription with every circadian cycle (under long photoperiods, there is no suppression of eya3 transcription, given the absence of melatonin in the morning [see Fig. 20]).
Dardente H, Wyse CA, Birnie MJ, Dupré SM, Loudon ASI, Lincoln GA, Hazlerigg DG. A molecular switch for photoperiod responsiveness in mammals. Curr Biol 20:2193-2198, 2010 [doi.org/10.1016/j.cub.2010.10.048].
Masumoto K, Ukai-Tadenuma M, Kasukawa T, Nagano M, Uno KD, Tsujino K, Horikawa K, Shigeyoshi Y, Ueda HR. Acute induction of Eya3 by late-night light stimulation triggers TSHβ expression in photoperiodism. Curr Biol 20:2199-2206, 2010 [doi.org/10.1016/j.cub.2010.11.038].
With regard to the seasonal regulation of reproduction, T3 does not act directly on gonadotropin-releasing hormone neurons but instead through distinct populations of neurons that express RFamide peptides (kisspeptins and RF-amide related peptides). Notably, these are expressed in hypothalamic nuclei involved in a range of metabolically-relevant functions, including food intake, fat mass regulation, and body temperature.
For review, see:
Henningsen JB, Gauer F, Simonneaux V. RFRP neurons – the doorway to understanding seasonal reproduction in mammals. Front Endocrinol 7:36, 2016 [doi.org/10.3389/fendo.2016.00036].
Soay sheep have served as a key large-animal model for elaborating the pre-eminent role of the pars tuberalis in seasonal timekeeping. They can be bred under controlled lighting regimens, and their size allows for precise surgical procedures around the hypothalamus and pituitary gland and for multiple blood sampling, including from portal capillaries of the median eminence. In hypothalamo-pituitary disconnected Soay rams – in which the pituitary gland is surgically isolated from all hypothalamic neural inputs – a direct action of melatonin on the pars tuberalis could be demonstrated, as assayed by cyclic prolactin secretion from adjacent pars distalis lactotrophs and by the timing of the pelage (hair) molt (Lincoln et al., 2006).
Lincoln GA, Clarke IJ, Hut RA, Hazlerigg DG. Characterizing a mammalian circannual pacemaker. Science 134:1941-1944, 2006 [doi.org/10.1126/science.1132009].
Some of the recent excitement about circalunar and circatidal clocks can be appreciated in recent reviews (Rock et al., 2022; Häfker et al., 2023; Ritter and Tessmar-Raible, 2024), a role for bmal1 in circatidal rhythms of crustacean model organisms (Kwiatkowski et al., 2023; Zhang et al., 2023), and natural observations in humans (Casiraghi et al., 2021; Helfrich-Förster et al., 2021; Wehr and Helfrich-Förster, 2021).
Rock A, Wilcockson D, Last KS. Towards an understanding of circatidal clocks. Front Physiol 13:830107, 2022 [doi.org/10.3389/fphys.2022.830107].
Häfker NS, Andreatta G, Manzotti A, Falciatore A, Raible F, Tessmar-Raible K. Rhythms and clocks in marine organisms. Ann Rev Mar Sci 15:509-538, 2023 [doi.org/10.1146/annurev-marine-030422-113038].
Ritter A, Tessmar-Raible T. Time me by the moon: The evolution and function of lunar timing systems. EMBO reports 25:3169-3176, 2024 [doi.org/10.1038/s44319-024-00196-5].
Kwiatkoski ER, Schnytzer Y, Rosenthal JJC, Emery P. Behavioral circatidal rhythms require Bmal1 in Parhyale hawaiensis. Curr Biol 33:1867-1882, 2023 [doi.org/10.1016/j.cub.2023.03.015].
Zhang L, Green EW, Webster SG, Hastings MH, Wilcockson DC, Kyriacou CP. The circadian clock gene bmal1 is necessary for co-ordinated circatidal rhythms in the marine isopod Eurydice pulchra (Leach). PLoS Genet 19: e1011011, 2023 [doi.org/10.1371/journal.pgen.1011011].
Casiraghi L, Spiousas I, Dunster GP, McGlothlen K, Fernández-Duque E, Valeggia C, de la Iglesia HO. Moonstruck sleep: Synchronization of human sleep with the moon cycle under field conditions. Sci Adv 7:eabe0465, 2021 [doi.org/10.1126/sciadv.abe0465].
Helfrich-Förster C, Monecke S, Spiousas I, Hovestadt T, Mitesser O, Wehr TA. Women temporarily synchronize their menstrual cycles with the luminance and gravimetric cycles of the moon. Sci Adv 7:eabe1358, 2021 [doi.org/10.1126/sciadv.abe1358].
Wehr TA, Helfrich-Förster C. Longitudinal observations call into question the scientific consensus that humans are unaffected by lunar cycles. BioEssays 43:2100054, 2021 [doi.org/10.1002/bies.202100054].
Gustav Kramer (1910 – 1959) observed that European starlings (Sturnus vulgaris) housed in an outdoor aviary became increasingly active (“restless”) at the time of their usual spring migration, orienting themselves in a direction appropriate to the season. So inspired, he devised a circular cage and, by the ingenious use of mirrors that deflected the apparent position of the sun in the sky, re-directed the birds’ orientation (Kramer, 1950). The obvious fact that the sun changes its position over time means that the birds must be able to compensate for that motion by an endogenous sense of the time of day. Further experiments by Kramer and others (Kramer, 1952; see Hoffmann, 1960) confirmed that the azimuth component of the sun’s position was the relevant daytime visual clue and also that the innate sun compass was being compensated for the sun’s predicted movement over time by an endogenous circadian mechanism. Thus, a shift of the light-dark cycle by 6 h re-oriented the birds’ direction by 90°; while illumination of the cage by a stationary (fixed) artificial “sun” – but interpreted by the birds as a moving sun – rotated their directional orientation by about 15° every hour.
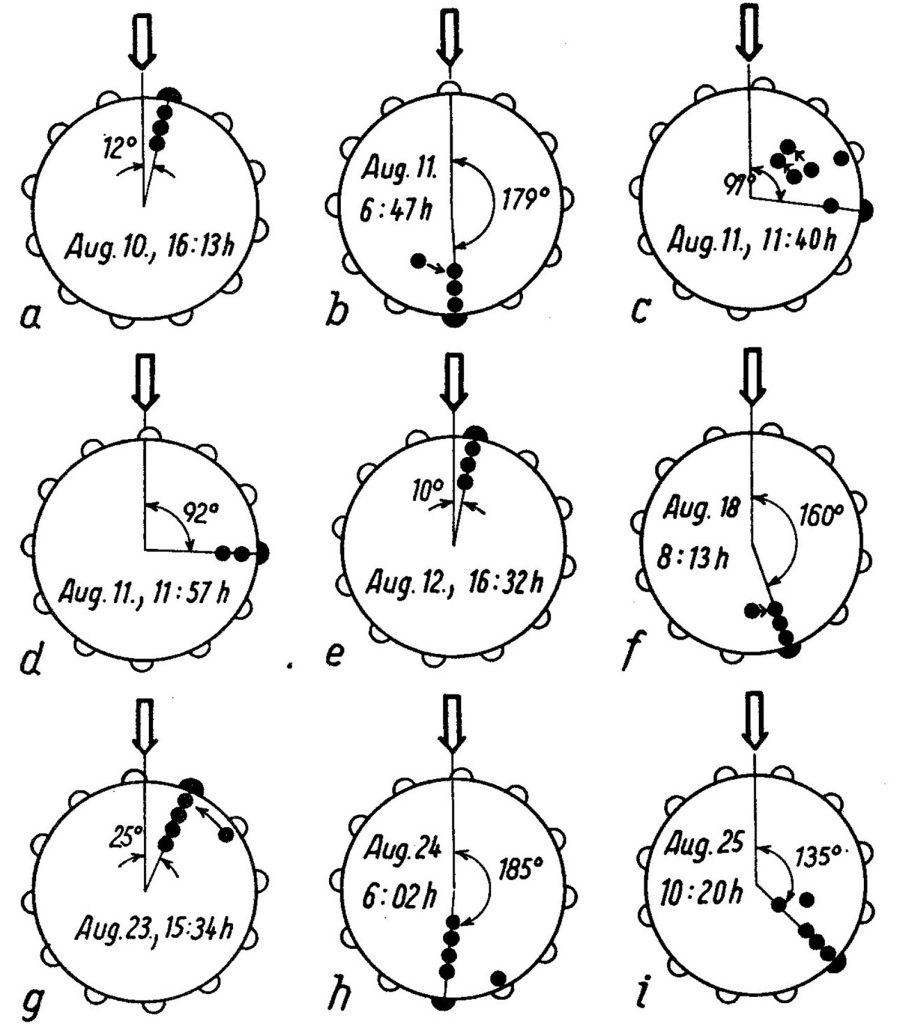
By permission, from Fig. 2 in: Hoffmann K. Experimental manipulation of the orientational clock in birds. Cold Spring Harb Symp Quant Biol 25:379-387, 1960 [doi.org/10.1101/SQB.1960.025.01.040].
Kramer G. Weitere Analyse der Faktoren, welche die Zugaktivität des gekäfigten Vogels orlentieren. Naturwiss 37:377-378, 1950 [doi.org/10.1007/BF00626007].
Kramer G. Experiments on bird orientation. Ibis 94:265-285, 1952 [doi.org/10.1111/j.1474-919X.1952.tb01817.x].
For reviews, see:
Merlin C, Iiams SE, Lugena AB. Monarch butterfly migration moving into the genetic era. Trends Genetics 36:689-701, 2020 [doi.org/10.1016/j.tig.2020.06.011].
Freedman MG, Kronforst MR. Migration genetics take flight: genetic and genomic insights into monarch butterfly migration. Curr Opin Insect Sci 59:101079, 2023 [doi.org/10.1016/j.cois.2023.101079].
Time-place learning, also known as time-place discrimination or time-place association, serves to link pivotal events with their specific spatial locations and times of day. Mechanistic analyses have been complicated, because in addition to circadian-based memory “time stamps,” there are other possible non-circadian mechanisms that can underlie this ability (such as recognizing contextual cues, memorizing sequences of events, or tracking short intervals of time) (for review, see Mulder et al., 2013). The first, and perhaps most famous, example of circadian time-place learning is the “time memory” (Zeitgedächtnis) in the foraging behavior of honeybees (Beling, 1929; Renner, 1960; Bloch et al., 2017) that involves a time-compensated sun compass (von Frisch and Lindauer, 1956). While circadian mechanisms are also involved in examples of mammalian time-place learning, the neurobiological basis remains uncertain (Mulder et al., 2014; Cole et al., 2016)
Mulder CK, Gerkema MP, Van der Zee EA. Circadian clocks and memory: time-place learning. Front Molec Neurosci 6:8, 2013 [doi.org/10.3389/fnmol.2013.00008].
Beling I. Über das Zeitgedächtnis der Bienen. Z Vergl Physiol 9:259-338, 1929 [doi.org/10.1007/BF00340159].
Renner M. The contribution of the honey bee to the study of time-sense and astronomical orientation. Cold Spring Harb Symp Quant Biol 25:361-367, 1960 [doi.org/10.1101/SQB.1960.025.01.037].
Bloch G, Bar-Shai N, Cytter Y, Green R. Time is honey: circadian clocks of bees and flowers and how their interactions may influence ecological communities. Phil Trans R Soc B 372:20160256, 2017 [doi.org/10.1098/rstb.2016.0256].
von Frisch K, Lindauer M. The “language” and orientation of the honey bee. Annu Rev Entomol 1:45–58, 1956 [doi.org/10.1146/annurev.en.01.010156.000401].
Mulder CK, Papantoniou C, Gerkema MP, Van der Zee EA. Neither the SCN nor the adrenals are required for circadian time-place learning in mice. Chronobiol Int 31:1075-1092, 2014 [doi.org/10.3109/07420528.2014.944975].
Cole E, Mistlberger RE, Merza D, Trigiani LJ, Madularu D, Simundic A, Mumby DG. Circadian time-place (or time-route) learning in rats with hippocampal lesions. Neurobiol Learning Memory 136:236-243, 2016 [doi.org/10.1016/j.nlm.2016.09.004].
For review, see:
Bloch G, Barnes BM, Gerkema MP, Helm B. Animal activity around the clock with no overt circadian rhythms: patterns, mechanisms and adaptive value. Proc R Soc B 280:20130019, 2013 [doi.org/10.1098/rspb.2013.0019].
See references:
Lu W, Meng Q-J, Tyler NJC, Stokkan K-A, Loudon ASI. A circadian clock is not required in an Arctic mammal. Curr Biol 20:533-537, 2010 [doi.org/10.1016/j.cub.2010.01.042].
Arnold W, Ruf T, Loe LE, Irvine RJ, Ropstad E, Veiberg V, Albon SD. Circadian rhythmicity persists through the polar night and midnight sun in Svalbard reindeer. Sci Rep 8:14466, 2018 [doi.org/10.1038/s41598-018-32778-4].
Reierth E, Stokkan K-A. Activity rhythm in high Arctic Svalbard ptarmigan (Lagopus mutus hyperboreus). Can J Zool 76:2031-2039, 1998 [doi.org/10.1139/z98-173].
Appenroth D, Wagner GC, Hazlerigg DG, West AC. Evidence for circadian-based photoperiodic timekeeping in Svalbard ptarmigan, the northernmost resident bird. Curr Biol 31:2720-2727, 2021 [doi.org/10.1016/j.cub.2021.04.009].