Chapter 3. APPLICATION: Consulting the Clock
Looking back on our Road Map, you will see that we have deconstructed, reconstructed, and peered into the adjustable moving parts of the circadian timing system. It functions as an innate ![]() 3.1, inherited, and adaptable timekeeping mechanism that coordinates rhythmic biological processes with each other and with predictable 24-h environmental changes.
3.1, inherited, and adaptable timekeeping mechanism that coordinates rhythmic biological processes with each other and with predictable 24-h environmental changes.
But is it a clock?
For this, we return one last time to our Oxford English Dictionary: clock (Middle English, likely mid-14th century) is “a device for the measurement and indication of the passage of time.” So, do organisms consult their circadian clocks as reference tools for marking the local time and measuring the passage of time, in addition to their role in temporal organization? The answer is a resounding yes.
***
Adapting to other consequential geophysical cycles
Of course, as the Earth is rotating daily on its axis it is also orbiting annually around the sun. Because the Earth’s axis remains tilted in a fixed direction (at 23.44°) throughout its entire orbit, the northern and southern hemispheres are alternately directed towards and away from the sun for half a year each, resulting in the seasons (Fig. 18).
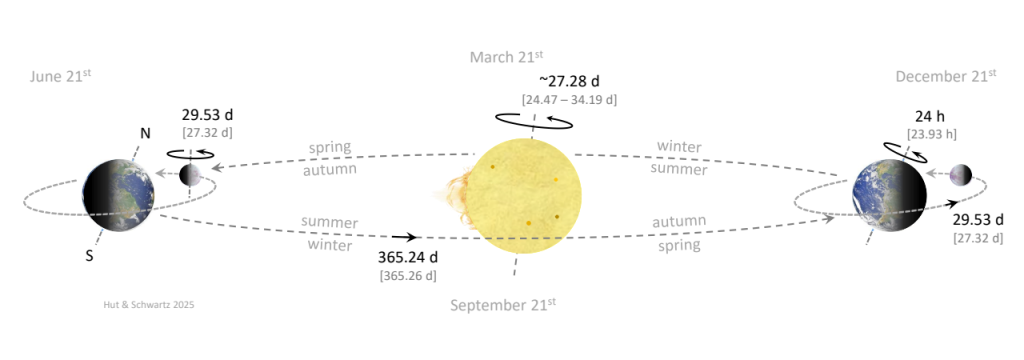
All axial rotation and orbital periods are as viewed from Earth (synodic), to suit our biological interests. Corresponding periods relative to the “fixed” position of the stars are indicated in brackets (sidereal). Synodic and sidereal periods differ because the Earth orbits the sun (precession effect). The Earth rotates around its axis in 24 h (that is, the average time it takes for the sun to reach its highest position in the sky again the next day; the synodic or solar day). The Moon orbits the Earth, and it takes 29.53 d from one full moon till the next (synodic month). Due to tidal friction, the Moon’s axial rotation became phase locked with its orbit around Earth, resulting in a synodic rotation period also of 29.53 d, causing the Moon to face the Earth always with the same side. Because the sun is not a solid body but a plasma, its sidereal rotation decreases with latitude (24.47 d at the equator and 34.19 d at the poles). Sunspots observed from Earth rotate with an average synodic period of ~27.28 d. The Earth takes 365.24 d to complete one orbit around the sun (synodic, tropical or solar year). Because of the Earth’s 23.44o axis tilt, the northern hemisphere is tilted towards the sun around June 21st (summer solstice, longest day length), while the southern hemisphere is tilted away from the sun (winter solstice, shortest day length). On December 21st, the situation is reversed (southern hemisphere: summer solstice, northern hemisphere: winter solstice). The progression of the seasons for the northern hemisphere is therefore in anti-phase with the southern hemisphere, as indicated respectively above and below the dashed orbital path of Earth.
Source: Hiesinger H, Jaumann J, in Encyclopedia of the Solar System (Third Edition), 2014. https://en.wikipedia.org/wiki/Rotation_period_(astronomy)
And seasonal changes in photoperiod (often referred to as “daylength,” that is, the length of the light phase) lead to large seasonal differences in ambient temperature, precipitation, and nutrient availability – all of which challenge anticipation and adaptation for life in temperate latitudes. These are met with dramatic annual cycles in reproduction, growth, body weight and composition, thermogenesis, morphology, and behavior. For some small nocturnal mammals, winter adaptation may even include switching to ultradian rhythmicity or being active during the diurnal temporal niche, since activity shifted to the higher ambient temperatures of the light phase should reduce overall energy expenditure ![]() 3.2; others may minimize their energy demands during winter through states of suspended animation (torpor and hibernation). The cycling of the seasons presents an additional critical problem: optimizing survival of the next generation, which is best if birth is timed to the spring, with plentiful food for growth and development of the young. This means that the anticipated seasonal window for mating will differ depending on the length of gestation. For small mammals (as in hamsters) with a short gestation lasting weeks (and also for birds like Japanese quail), breeding occurs in early spring as the light phase lengthens (and the dark phase shortens), and they are referred to as “long day” (LD) (or better, long photoperiod [LP]) breeders; for large mammals (as in sheep, deer) with a long gestation lasting months, breeding occurs in autumn as the light phase shortens (and the dark phase lengthens), and they are referred to as “short day” (SD) (or better, short photoperiod [SP]) breeders. Reproductive competence is triggered when the change of photoperiod reaches a critical duration, depending on species, latitude, temperature, food intake, and, most importantly, prior photoperiodic history
3.2; others may minimize their energy demands during winter through states of suspended animation (torpor and hibernation). The cycling of the seasons presents an additional critical problem: optimizing survival of the next generation, which is best if birth is timed to the spring, with plentiful food for growth and development of the young. This means that the anticipated seasonal window for mating will differ depending on the length of gestation. For small mammals (as in hamsters) with a short gestation lasting weeks (and also for birds like Japanese quail), breeding occurs in early spring as the light phase lengthens (and the dark phase shortens), and they are referred to as “long day” (LD) (or better, long photoperiod [LP]) breeders; for large mammals (as in sheep, deer) with a long gestation lasting months, breeding occurs in autumn as the light phase shortens (and the dark phase lengthens), and they are referred to as “short day” (SD) (or better, short photoperiod [SP]) breeders. Reproductive competence is triggered when the change of photoperiod reaches a critical duration, depending on species, latitude, temperature, food intake, and, most importantly, prior photoperiodic history ![]() 3.3.
3.3.
This kind of anticipatory phenotypic switch hints at the possible existence of an endogenous circa-annual oscillator. The search for this putative oscillator has been a challenging enterprise: demonstrating free-running circannual rhythms requires measurements over several cycles (years) in constant conditions, and many of our favorite model species are short-lived and their breeding is completed after one season ![]() 3.4. Nevertheless, circannual rhythms in a variety of long-lived species (maintained under a fixed photoperiod, usually a 12 h: 12 h light-dark cycle) have been clearly documented, including rhythms in body weight, reproduction, hibernation, and molting (Fig. 19). Generally, free-running circannual periods are in the range of 10 – 11 months.
3.4. Nevertheless, circannual rhythms in a variety of long-lived species (maintained under a fixed photoperiod, usually a 12 h: 12 h light-dark cycle) have been clearly documented, including rhythms in body weight, reproduction, hibernation, and molting (Fig. 19). Generally, free-running circannual periods are in the range of 10 – 11 months.
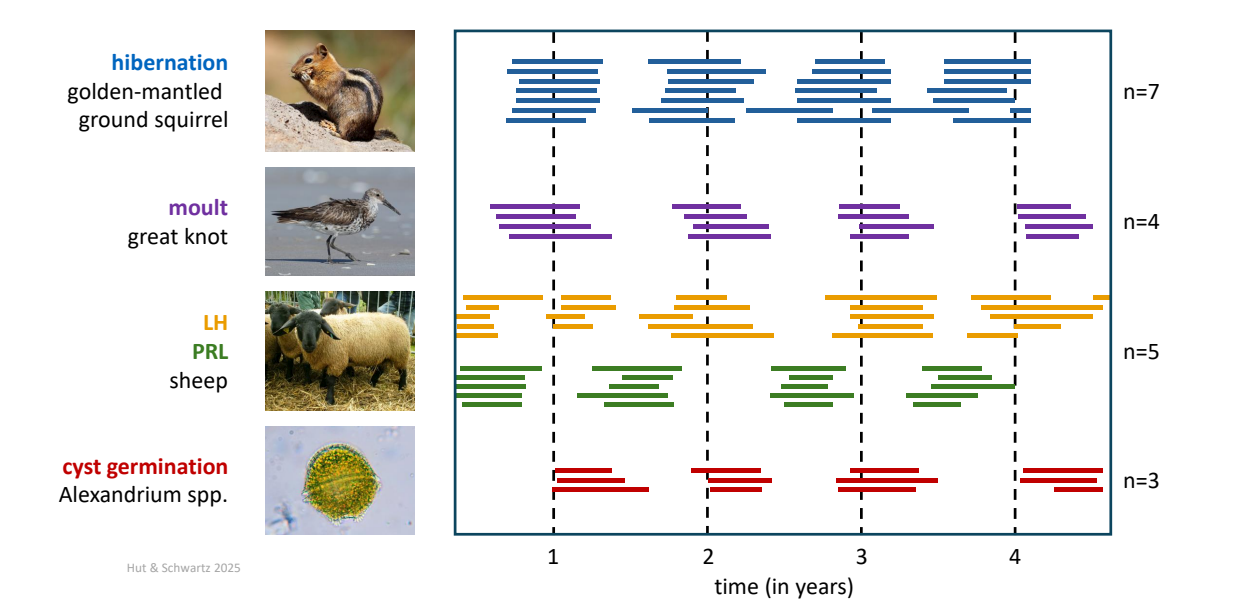
Data collected over four years; vertical dashed lines represent January 1 of each year.
Stacked blue bars: timing of hibernation in seven individual golden mantled ground squirrels (Callospermophilus lateralis) under constant conditions (12 h: 12 h light-dark cycle; temperature 3oC).
Stacked purple bars: timing of moult in four individual great knots (Calidris tenuirostris) under constant conditions (12 h: 12 h light-dark cycle; temperature 15oC).
Stacked orange and green bars: timing of high serum luteinizing hormone (LH) and prolactin (PRL) concentrations, respectively, in five individual sheep (Suffolk ewes) under constant lighting conditions (8 h :16 h light-dark cycle, temperature not regulated).
Stacked red bars: timing of cyst germination in three populations of dinoflagellate algae (Alexandrium spp.) under constant conditions (constant darkness; temperature 2oC).
Data from:
Squirrel: Pengelley ET, Asmundson SJ, Barnes B, Aloia RC. Relationship of light intensity and photoperiod to circannual rhythmicity in the hibernating ground squirrel, Citellus lateralis. Comp Biochem Physiol 53:273-277, 1976 [doi.org/10.1016/S0300-9629(76)80035-7].
Great knot: Piersma T, Brugge M, Spaans B, Battley PF. Endogenous circannual rhythmicity in body mass, molt, and plumage of great knots (Calidris tenuirostris). The Auk 125:140-148, 2008 [doi.org/10.1525/auk.2008.125.1.140].
Sheep: Karsch FJ, Robinson JE, Woodfill CJI, Brown MB. Circannual cycles of luteinizing hormone and prolactin secretion in ewes during prolonged exposure to a fixed photoperiod: Evidence for an endogenous reproductive rhythm. Biol Reprod 41:1034-1046, 1989 [doi.org/10.1095/biolreprod41.6.1034].
Alexandrium spp.: Matrai P, Thompson B, Keller M. Circannual excystment of resting cysts of Alexandrium spp. from eastern Gulf of Mexico populations. Deep Sea Res 52:2560-2568, 2005 [doi.org/10.1016/j.dsr2.2005.06.013].
Photo credits:
Squirrel: © Frank Schulenburg / CC BY-SA 4.0,
https://commons.wikimedia.org/wiki/File:Callospermophilus_lateralis_near_Lake_Almanor.jpg
Great knot: Sunphlo, South Australia – Calidris tenuirostris / Great Knot, CC BY-SA 2.0,
https://commons.wikimedia.org/w/index.php?curid=36492740
Sheep: Saruman – Own work, Public Domain,
https://commons.wikimedia.org/w/index.php?curid=2839618
Alexandrium: A. minutum by Philippe Garcelon,
https://www.flickr.com/photos/78178083@N05/51841087009/, CC BY 2.0, https://commons.wikimedia.org/w/index.php?curid=139183910
Of all the potential Zeitgebers for the accurate yearly entrainment of circannual clocks, the most powerful and predictive would seem to be the seasonal change in photoperiod. It was in the 1930’s that the circadian clock was proposed as the underlying mechanism for photoperiodic time measurement; over the next few decades it was experimentally implicated through stepwise manipulation of dark phase duration or by interruption of the dark phase with light pulses applied at different times after dark onset ![]() 3.5. You may have come across the terms “external” and “internal” coincidence. These were ideas for how the changing photoperiod might be detected by the circadian oscillator: the former suggested photoperiodic variation of illumination on a light-sensitive phase of the clock’s oscillation, while the latter posited variation of the phase relationship between two coupled circadian oscillators (or sets of oscillators) individually entrained to dawn and dusk. At least for mammals, we now know that the SCN encodes photoperiod by a reconfiguration of the phases of individual oscillating SCN cells (perhaps as part of an “internal” coincidence concept), adopting wide and narrow distributions across long and short photoperiods, respectively
3.5. You may have come across the terms “external” and “internal” coincidence. These were ideas for how the changing photoperiod might be detected by the circadian oscillator: the former suggested photoperiodic variation of illumination on a light-sensitive phase of the clock’s oscillation, while the latter posited variation of the phase relationship between two coupled circadian oscillators (or sets of oscillators) individually entrained to dawn and dusk. At least for mammals, we now know that the SCN encodes photoperiod by a reconfiguration of the phases of individual oscillating SCN cells (perhaps as part of an “internal” coincidence concept), adopting wide and narrow distributions across long and short photoperiods, respectively ![]() 3.6. In this way, the duration of the dark phase (or of the subjective night in constant darkness) is accurately reflected in the duration of elevated nocturnal pineal melatonin levels – both in diurnal and nocturnal animals and in long- and short-photoperiod breeders – creating a faithful photo-neuro-endocrine signal transduction pathway (Fig. 20).
3.6. In this way, the duration of the dark phase (or of the subjective night in constant darkness) is accurately reflected in the duration of elevated nocturnal pineal melatonin levels – both in diurnal and nocturnal animals and in long- and short-photoperiod breeders – creating a faithful photo-neuro-endocrine signal transduction pathway (Fig. 20).
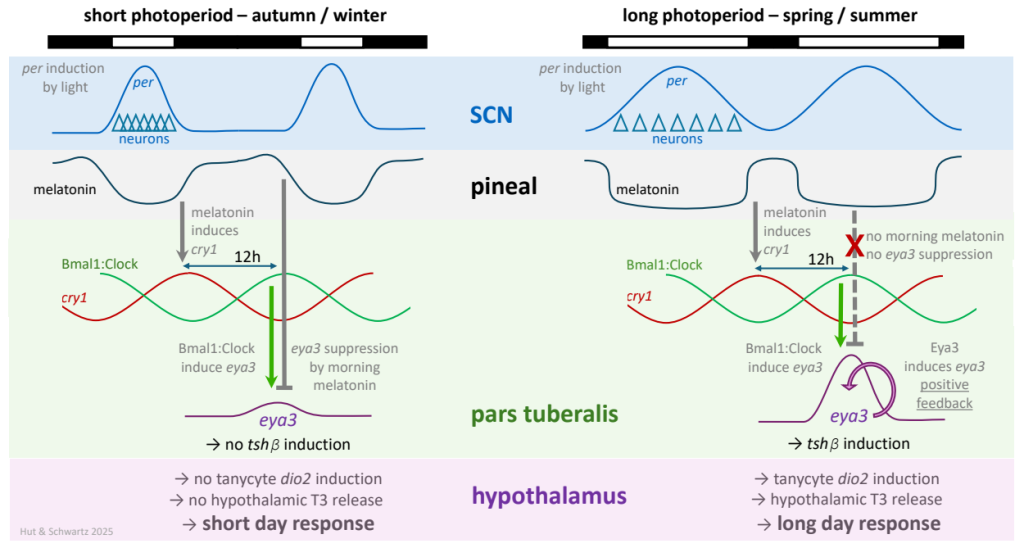
Photic induction of SCN per genes leads to the entrainment of the rhythm of SCN electrical activity, with compressed (left) and expanded (right) durations of high activity in short and long photoperiods, respectively (see ![]() 3.6); as a result, the duration of high pineal melatonin levels during the dark phase is long in the short photoperiod and short in the long photoperiod. Under both photoperiods, plasma melatonin onset induces cry1 in pars tuberalis cells, entraining their circadian clocks to evening lights-off; 12 h later, Bmal1:Clock heterodimers induce eya3 (a transcriptional co-activator; see
3.6); as a result, the duration of high pineal melatonin levels during the dark phase is long in the short photoperiod and short in the long photoperiod. Under both photoperiods, plasma melatonin onset induces cry1 in pars tuberalis cells, entraining their circadian clocks to evening lights-off; 12 h later, Bmal1:Clock heterodimers induce eya3 (a transcriptional co-activator; see ![]() 3.7). Under the short photoperiod, the presence of melatonin in the morning suppresses further eya3 transcription; but under the long photoperiod (with night lengths shorter than 12 h), the absence of melatonin in the morning allows positive feedback of the Eya3 protein to reinforce its own transcription. Once photoperiod length has surpassed a critical duration (critical photoperiod), augmented Eya3 expression subsequently activates TSHβ transcription. The ensuing cascade involves the stimulation of ependymal tanycytes to upregulate deiodinase type 2 (dio2) and downregulate dio3, thereby activating thyroid hormone conversion from T4 → T3 in the hypothalamus, and ultimately a long day response phenotype.
3.7). Under the short photoperiod, the presence of melatonin in the morning suppresses further eya3 transcription; but under the long photoperiod (with night lengths shorter than 12 h), the absence of melatonin in the morning allows positive feedback of the Eya3 protein to reinforce its own transcription. Once photoperiod length has surpassed a critical duration (critical photoperiod), augmented Eya3 expression subsequently activates TSHβ transcription. The ensuing cascade involves the stimulation of ependymal tanycytes to upregulate deiodinase type 2 (dio2) and downregulate dio3, thereby activating thyroid hormone conversion from T4 → T3 in the hypothalamus, and ultimately a long day response phenotype.
Great progress is now being made in dissecting the anatomical, biochemical, and molecular substrates downstream of the melatonin rhythm that underlie seasonal physiological responses. Attention has been directed especially to the pars tuberalis as a key element in birds and mammals, in large part because it is the only nervous system structure that consistently expresses melatonin receptors across a wide range of long- and short-photoperiod breeders. As a tubular structure that wraps around the pituitary stalk, the pars tuberalis is not only in direct contact with the portal vessels of the median eminence but also lies adjacent to the anterior pituitary gland (pars distalis). Both the pars tuberalis and the pars distalis contain cells that release thyroid stimulating hormone (TSH), but their regulation is distinctly different. Pars tuberalis thyrotropes do not express triiodothyronine (T3) or thyroid releasing hormone receptors and are thus not a part of the classic hypothalamo-pars distalis-thyroid feedback axis; instead, TSHβ secretion in the pars tuberalis is regulated by a melatonin-dependent network of circadian clock and clock-controlled genes (the emerging details of which are perhaps akin to an “external” coincidence concept ![]() 3.7)(Fig. 20). TSH-receptor-bearing ependymal tanycytes lining the third ventricle then control the levels of the bioactive thyroid hormone T3 in the mediobasal hypothalamus through enzymatic processing by deiodinases. The distal mechanisms responsible for species-specific differential responses, and for integrating the reproductive, behavioral, and metabolic components of the seasonal phenotype, are under active investigation
3.7)(Fig. 20). TSH-receptor-bearing ependymal tanycytes lining the third ventricle then control the levels of the bioactive thyroid hormone T3 in the mediobasal hypothalamus through enzymatic processing by deiodinases. The distal mechanisms responsible for species-specific differential responses, and for integrating the reproductive, behavioral, and metabolic components of the seasonal phenotype, are under active investigation ![]() 3.8, as are the identit(ies) of hypothesized paracrine factor(s) from the pars tuberalis that regulate circannual prolactin secretion by pars distalis lactotrophs
3.8, as are the identit(ies) of hypothesized paracrine factor(s) from the pars tuberalis that regulate circannual prolactin secretion by pars distalis lactotrophs ![]() 3.9.
3.9.
By the way, there is an additional geophysical force affecting life on Earth – the Moon’s orbit around the planet, which gives rise to multiple astronomical cycles and obvious environmental consequences that are exceedingly complex. The lunar cycle of 29.53 days (the synodic month; Fig. 18) is the interval of time for the Moon to orbit the Earth, progressing through phases of moonlight intensity from maximum (full moon) to minimum (new moon) defined by illumination from the sun. The lunidian cycle of 24.8 h (the lunar “day”) is the interval of time between consecutive moonrises as viewed on Earth, the gravitational consequences of which help shape the ocean tides (the 12.4 h tidal cycle, with two high and two low tides per lunar day). The lunar cycle also contributes to the periodicity of the tides: twice a synodic month (that is, the semilunar cycle ), the sun, Earth, and Moon are all aligned at the full and new moons, creating an increased gravitational force that generates a higher high tide and lower low tide than normal (spring tides) (this in contrast to the decreased gravitational force at the first and last quarter moons [neap tides]). Note that there are additional levels of complexity shaping tidal phenomena, due to the Moon’s elliptical orbit and its declination relative to the plane of the Earth’s equator.
While monthly reproductive rhythms and intertidal behavioral rhythms have been appreciated since antiquity, only recently has there been a resurgence of interest in the possible role of endogenous circa-oscillators entrained by rhythms in the intensity of moonlight or the rise and fall of the tides. Research has been fueled by links to circadian clock gene homologs and rhythms in diverse marine organisms, transcriptome analyses, and longitudinal studies of human physiology and behavior (the latter, for example, on menstrual cycles and bipolar disorder) ![]() 3.10.
3.10.
***
Wayfinding on a rotating world
There exists another remarkable strategy for adapting to the seasons. Several species of birds (and marine mammals) undertake round-trip migrations every spring and autumn to and from their breeding grounds; some involve long distance routes to destinations thousands of miles and months away, with future local conditions at the target sites unknown at the time of departure. Such a mobile lifestyle relies on both circannual and circadian temporal programs. For timing of migration, anticipatory physiological and morphological preparations for the journey are timed by a photoperiod-entrained circannual clock, culminating in a departure time that can be studied in captive birds by the onset of near constant 24-h locomotor activity (termed Zugunruhe, or “migratory restlessness”). For orientation during migration, birds likely exploit multiple navigational cues while in flight, but one is based on the position of the sun. Early in the 1950’s, studies of captive European starlings demonstrated the existence of an innate sun compass necessarily adjusted for the passage of time by a circadian mechanism ![]() 3.11. Notably, outside the laboratory, such a time-compensated sun-compass mechanism might face some interesting challenges; as an example, for trans-meridian flight paths at very high latitudes, is there a mechanism for rapid, repeated resetting of the circadian system to local solar time (a jet-lagged sun compass is surely not a functional device)? Eventually, along the way, flexible re-calibration of the itinerary is needed in response to homeostatic demands (for example, in the timing of flight segments and stopovers based on energy reserves) and environmental cues (for example, in setting arrival and breeding times independently based on local conditions). And eventually there is the final challenge of the return trip, now using the compass to plot a reversed path. At least in monarch butterflies, ambient temperature plays a key role in the directional switch. These insects complete a trans-generational migration between North America and their overwintering grounds in Mexico, and winter cold exposure appears to be required for northward reorientation in the spring
3.11. Notably, outside the laboratory, such a time-compensated sun-compass mechanism might face some interesting challenges; as an example, for trans-meridian flight paths at very high latitudes, is there a mechanism for rapid, repeated resetting of the circadian system to local solar time (a jet-lagged sun compass is surely not a functional device)? Eventually, along the way, flexible re-calibration of the itinerary is needed in response to homeostatic demands (for example, in the timing of flight segments and stopovers based on energy reserves) and environmental cues (for example, in setting arrival and breeding times independently based on local conditions). And eventually there is the final challenge of the return trip, now using the compass to plot a reversed path. At least in monarch butterflies, ambient temperature plays a key role in the directional switch. These insects complete a trans-generational migration between North America and their overwintering grounds in Mexico, and winter cold exposure appears to be required for northward reorientation in the spring ![]() 3.12.
3.12.
Lastly, on a spatial scale quite different from wayfinding around the globe – but equally critical to survival in the wild – is wayfinding around the neighborhood. On a rotating world, resources may become available in particular locations at specific times of the day and night – as well as the presence of predators – and learning to visit or avoid such sites at the right times (time-place learning) is believed to be a strategy to optimize energy expended in the search, minimizing futile or dangerous forays. This ability has been described in a number of animals – in certain species of insects, birds, and mammals – and at least in some cases a role for the circadian system in time measurement has been demonstrated ![]() 3.13.
3.13.
***
A final word – On opting out
Although we (the authors) are clearly unabashed champions of living in harmony with one’s circadian clock, even we must admit that there could be circumstances when going off the clock might be the right thing to do. This is apparently so, as indicated by fascinating natural examples of animals that exhibit plastic locomotor activity patterns ![]() 3.14. We have already mentioned the switch from diurnal to near-constant activity during seasonal avian migrations, a change also reported in a polygynous Arctic shorebird (pectoral sandpiper) for a few weeks during the continuous light of the summer breeding season. In eusocial insects like ants, honeybees, and bumblebees, workers adopt locomotor regimens congruent with their assigned tasks, uninterrupted if caring (“nursing”) the brood in the nest but strongly diurnal if foraging outside. Bumblebee queens without and with brood alternate between circadian and continuous activity levels, respectively, and the switch can even anticipate the arrival of brood.
3.14. We have already mentioned the switch from diurnal to near-constant activity during seasonal avian migrations, a change also reported in a polygynous Arctic shorebird (pectoral sandpiper) for a few weeks during the continuous light of the summer breeding season. In eusocial insects like ants, honeybees, and bumblebees, workers adopt locomotor regimens congruent with their assigned tasks, uninterrupted if caring (“nursing”) the brood in the nest but strongly diurnal if foraging outside. Bumblebee queens without and with brood alternate between circadian and continuous activity levels, respectively, and the switch can even anticipate the arrival of brood.
Essentially unknown are the mechanisms responsible for shifting activity levels from rhythmic to constant over 24 h, but they probably do not involve a complete arrest of the entire circadian system; some network components may remain operational ![]() 3.15. Svalbard reindeer and ptarmigan (a member of the grouse family) are herbivorous arctic residents during the continuous light and dark of summer and winter, respectively. In reindeer, circadian regulation of melatonin rhythmicity is lost under these conditions, whereas locomotor activity remains weakly rhythmic; in ptarmigan, locomotor rhythmicity is lost, while circadian-based photoperiodic timing persists.
3.15. Svalbard reindeer and ptarmigan (a member of the grouse family) are herbivorous arctic residents during the continuous light and dark of summer and winter, respectively. In reindeer, circadian regulation of melatonin rhythmicity is lost under these conditions, whereas locomotor activity remains weakly rhythmic; in ptarmigan, locomotor rhythmicity is lost, while circadian-based photoperiodic timing persists.
With new and developing technologies that allow for remote, multi-modal, real-time rhythm tracking as well as the expansion of our armamentarium of “model” species, the significance of cases like these will not be as zoological curiosities but as exciting opportunities for better understanding the diversity and dynamic architectures of animal circadian clockworks and their role in sustaining the diversity of life.
